Dynamic requirements for a functional protein hinge
- PMID: 17336327
- PMCID: PMC2203303
- DOI: 10.1016/j.jmb.2007.01.074
Dynamic requirements for a functional protein hinge
Abstract
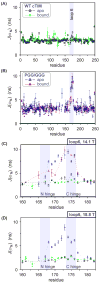
The enzyme triosephosphate isomerase (TIM) is a model of catalytic efficiency. The 11 residue loop 6 at the TIM active site plays a major role in this enzymatic prowess. The loop moves between open and closed states, which facilitate substrate access and catalysis, respectively. The N and C-terminal hinges of loop 6 control this motion. Here, we detail flexibility requirements for hinges in a comparative solution NMR study of wild-type (WT) TIM and a quintuple mutant (PGG/GGG). The latter contained glycine substitutions in the N-terminal hinge at Val167 and Trp168, which follow the essential Pro166, and in the C-terminal hinge at Lys174, Thr175, and Ala176. Previous work demonstrated that PGG/GGG has a tenfold higher Km value and 10(3)-fold reduced k(cat) relative to WT with either d-glyceraldehyde 3-phosphate or dihyrdroxyacetone phosphate as substrate. Our NMR results explain this in terms of altered loop-6 dynamics in PGG/GGG. In the mutant, loop 6 exhibits conformational heterogeneity with corresponding motional rates <750 s(-1) that are an order of magnitude slower than the natural WT loop 6 motion. At the same time, nanosecond timescale motions of loop 6 are greatly enhanced in the mutant relative to WT. These differences from WT behavior occur in both apo PGG/GGG and in the form bound to the reaction-intermediate analog, 2-phosphoglycolate (2-PGA). In addition, as indicated by 1H, 15N and 13CO chemical-shifts, the glycine substitutions diminished the enzyme's response to ligand, and induced structural perturbations in apo and 2-PGA-bound forms of TIM that are atypical of WT. These data show that PGG/GGG exists in multiple conformations that are not fully competent for ligand binding or catalysis. These experiments elucidate an important principle of catalytic hinge design in proteins: structural rigidity is essential for focused motional freedom of active-site loops.
Figures
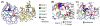






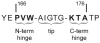
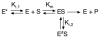
Similar articles
-
Entropy effects on protein hinges: the reaction catalyzed by triosephosphate isomerase.Biochemistry. 2004 Sep 14;43(36):11436-45. doi: 10.1021/bi049208d. Biochemistry. 2004. PMID: 15350130
-
Structural studies show that the A178L mutation in the C-terminal hinge of the catalytic loop-6 of triosephosphate isomerase (TIM) induces a closed-like conformation in dimeric and monomeric TIM.Acta Crystallogr D Biol Crystallogr. 2008 Feb;64(Pt 2):178-88. doi: 10.1107/S0907444907059021. Epub 2008 Jan 16. Acta Crystallogr D Biol Crystallogr. 2008. PMID: 18219118
-
Characterization of enzyme motions by solution NMR relaxation dispersion.Acc Chem Res. 2008 Feb;41(2):214-21. doi: 10.1021/ar700132n. Epub 2008 Feb 19. Acc Chem Res. 2008. PMID: 18281945 Review.
-
Role of loop-loop interactions in coordinating motions and enzymatic function in triosephosphate isomerase.Biochemistry. 2009 Jun 2;48(21):4548-56. doi: 10.1021/bi9002887. Biochemistry. 2009. PMID: 19348462 Free PMC article.
-
Triosephosphate isomerase: a highly evolved biocatalyst.Cell Mol Life Sci. 2010 Dec;67(23):3961-82. doi: 10.1007/s00018-010-0473-9. Epub 2010 Aug 7. Cell Mol Life Sci. 2010. PMID: 20694739 Free PMC article. Review.
Cited by
-
Loop 7 of E2 enzymes: an ancestral conserved functional motif involved in the E2-mediated steps of the ubiquitination cascade.PLoS One. 2012;7(7):e40786. doi: 10.1371/journal.pone.0040786. Epub 2012 Jul 18. PLoS One. 2012. PMID: 22815819 Free PMC article.
-
Millisecond Timescale Motions Connect Amino Acid Interaction Networks in Alpha Tryptophan Synthase.Front Mol Biosci. 2018 Nov 8;5:92. doi: 10.3389/fmolb.2018.00092. eCollection 2018. Front Mol Biosci. 2018. PMID: 30467546 Free PMC article.
-
Compensatory and long-range changes in picosecond-nanosecond main-chain dynamics upon complex formation: 15N relaxation analysis of the free and bound states of the ubiquitin-like domain of human plexin-B1 and the small GTPase Rac1.J Mol Biol. 2008 Apr 11;377(5):1474-87. doi: 10.1016/j.jmb.2008.01.081. Epub 2008 Feb 4. J Mol Biol. 2008. PMID: 18321527 Free PMC article.
-
Light chain somatic mutations change thermodynamics of binding and water coordination in the HyHEL-10 family of antibodies.Mol Immunol. 2009 Dec;47(2-3):457-64. doi: 10.1016/j.molimm.2009.08.018. Epub 2009 Sep 24. Mol Immunol. 2009. PMID: 19781789 Free PMC article.
-
A role for A-to-I RNA editing in temperature adaptation.Physiology (Bethesda). 2012 Dec;27(6):362-9. doi: 10.1152/physiol.00029.2012. Physiology (Bethesda). 2012. PMID: 23223630 Free PMC article. Review.
References
-
- Blacklow SC, Raines RT, Lim WA, Zamore PD, Knowles JR. Triosephosphate isomerase catalysis is diffusion controlled. Biochemistry. 1988;27:1158–1167. - PubMed
-
- Pompliano DL, Peyman A, Knowles JR. Stabilization of a reaction intermediate as a catalytic device: Definition of the functional role of the flexible loop in triosephosphate isomerase. Biochemistry. 1990;29:3186–3194. - PubMed
-
- Leszczynski JF, Rose GD. Loops in Globular-Proteins - a Novel Category of Secondary Structure. Science. 1986;234:849–855. - PubMed
-
- Fetrow JS. Protein Motifs. 6 Omega-Loops - Nonregular Secondary Structures Significant in Protein Function and Stability. FASEB J. 1995;9:708–717. - PubMed
-
- Davenport RC, Bash PA, Seaton BA, Karplus M, Petsko GA, Ringe D. Structure of the triosephosphate isomerase-phosphoglycohydroxamate complex: An analog of the intermediate on the reaction pathway. Biochemistry. 1991;30:5821–5826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

