Inhibiting pollen reduced nicotinamide adenine dinucleotide phosphate oxidase-induced signal by intrapulmonary administration of antioxidants blocks allergic airway inflammation
- PMID: 17336614
- PMCID: PMC3042252
- DOI: 10.1016/j.jaci.2006.11.634
Inhibiting pollen reduced nicotinamide adenine dinucleotide phosphate oxidase-induced signal by intrapulmonary administration of antioxidants blocks allergic airway inflammation
Abstract
Background: Ragweed extract (RWE) contains NADPH oxidases that induce oxidative stress in the airways independent of adaptive immunity (signal 1) and augment antigen (signal 2)-induced allergic airway inflammation.
Objective: To test whether inhibiting signal 1 by administering antioxidants inhibits allergic airway inflammation in mice.
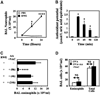
Methods: The ability of ascorbic acid (AA), N-acetyl cystenine (NAC), and tocopherol to scavenge pollen NADPH oxidase-generated reactive oxygen species (ROS) was measured. These antioxidants were administered locally to inhibit signal 1 in the airways of RWE-sensitized mice. Recruitment of inflammatory cells, mucin production, calcium-activated chloride channel 3, IL-4, and IL-13 mRNA expression was quantified in the lungs.
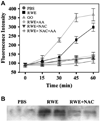
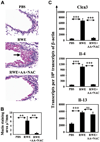
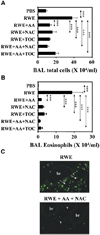
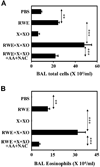
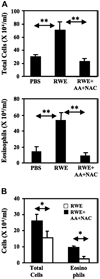
Results: Antioxidants inhibited ROS generation by pollen NADPH oxidases and intracellular ROS generation in cultured epithelial cells. AA in combination with NAC or Tocopherol decreased RWE-induced ROS levels in cultured bronchial epithelial cells. Coadministration of antioxidants with RWE challenge inhibited 4-hydroxynonenal adduct formation, upregulation of Clca3 and IL-4 in lungs, mucin production, recruitment of eosinophils, and total inflammatory cells into the airways. Administration of antioxidants with a second RWE challenge also inhibited airway inflammation. However, administration of AA+NAC 4 or 24 hours after RWE challenge failed to inhibit allergic inflammation.
Conclusion: Signal 1 plays a proinflammatory role during repeated exposure to pollen extract. We propose that inhibiting signal 1 by increasing antioxidant potential in the airways may be a novel therapeutic strategy to attenuate pollen-induced allergic airway inflammation.
Clinical implications: Administration of antioxidants in the airways may constitute a novel therapeutic strategy to prevent pollen induced allergic airway inflammation.
Conflict of interest statement
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
Figures

References
-
- Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F, et al. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L671–L679. - PubMed
-
- Hiltermann TJ, Peters EA, Alberts B, Kwikkers K, Borggreven PA, Hiemstra PS, et al. Ozone-induced airway hyperresponsiveness in patients with asthma: role of neutrophil-derived serine proteinases. Free Radic Biol Med. 1998;24:952–958. - PubMed
-
- Becker S, Soukup JM, Gallagher JE. Differential particulate air pollution induced oxidant stress in human granulocytes, monocytes and alveolar macrophages. Toxicol In Vitro. 2002;16:209–218. - PubMed
-
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. - PubMed
-
- Kagan V, Serbinova E, Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun. 1990;169:851–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

