The axon guidance gene lola is required for programmed cell death in the Drosophila ovary
- PMID: 17336958
- PMCID: PMC1905497
- DOI: 10.1016/j.ydbio.2007.01.029
The axon guidance gene lola is required for programmed cell death in the Drosophila ovary
Abstract
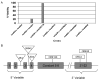
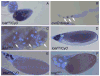
longitudinals-lacking (lola) was identified in Drosophila as a gene encoding several alternatively spliced transcription factors involved in axon guidance. Here we report that lola also plays a critical role in programmed cell death in the ovary. lola mutant germline clones show a high percentage of egg chambers with nurse cell nuclei persisting past stage 13, indicating a block in developmental nurse cell death. Mutants also show a disruption in the induced programmed cell death that occurs during mid-oogenesis in response to starvation. Further characterization revealed that lola germline clones exhibit abnormal nuclear organization which becomes increasingly severe with age. Chromatin appears diffuse and fails to condense properly or undergo DNA fragmentation in dying nurse cells. Masses of nuclear material accumulate in the ovaries of older flies containing lola germline clones. We propose that lola is necessary for complete chromatin condensation which occurs during programmed cell death in the ovary. Alleles differed in the strength of their phenotypes but interestingly, the severity of their ovarian phenotypes was independent of the strength of their neuronal phenotypes, suggesting a differential requirement for individual lola isoforms in the ovary and nervous system.
Figures

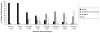
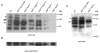

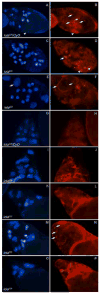
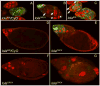

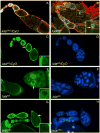
References
-
- Bao X, Zhang W, Krencik R, Deng H, Wang Y, Girton J, Johansen J, Johansen KM. The JIL-1 kinase interacts with lamin Dm0 and regulates nuclear lamina morphology of Drosophila nurse cells. J Cell Sci. 2005;118:5079–87. - PubMed
-
- Baum JS, St George JP, McCall K. Programmed cell death in the germline. Semin Cell Dev Biol. 2005;16:245–59. - PubMed
-
- Cavaliere V, Taddei C, Gargiulo G. Apoptosis of nurse cells at the late stages of oogenesis. Dev Genes Evol. 1998;208:106–112. - PubMed
-
- Cooley L, Verheyen E, Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992;69:173–184. - PubMed
-
- Crowner D, Madden K, Goeke S, Giniger E. Lola regulates midline crossing of CNS axons in Drosophila. Development. 2002;129:1317–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

