Iron acquisition by phytosiderophores contributes to cadmium tolerance
- PMID: 17337530
- PMCID: PMC1851820
- DOI: 10.1104/pp.106.094474
Iron acquisition by phytosiderophores contributes to cadmium tolerance
Abstract
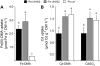
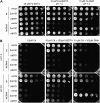
Based on the ability of phytosiderophores to chelate other heavy metals besides iron (Fe), phytosiderophores were suggested to prevent graminaceous plants from cadmium (Cd) toxicity. To assess interactions between Cd and phytosiderophore-mediated Fe acquisition, maize (Zea mays) plants were grown hydroponically under limiting Fe supply. Exposure to Cd decreased uptake rates of 59Fe(III)-phytosiderophores and enhanced the expression of the Fe-phytosiderophore transporter gene ZmYS1 in roots as well as the release of the phytosiderophore 2'-deoxymugineic acid (DMA) from roots under Fe deficiency. However, DMA hardly mobilized Cd from soil or from a Cd-loaded resin in comparison to the synthetic chelators diaminetriaminepentaacetic acid and HEDTA. While nano-electrospray-high resolution mass spectrometry revealed the formation of an intact Cd(II)-DMA complex in aqueous solutions, competition studies with Fe(III) and zinc(II) showed that the formed Cd(II)-DMA complex was weak. Unlike HEDTA, DMA did not protect yeast (Saccharomyces cerevisiae) cells from Cd toxicity but improved yeast growth in the presence of Cd when yeast cells expressed ZmYS1. When supplied with Fe-DMA as a Fe source, transgenic Arabidopsis (Arabidopsis thaliana) plants expressing a cauliflower mosaic virus 35S-ZmYS1 gene construct showed less growth depression than wild-type plants in response to Cd. These results indicate that inhibition of ZmYS1-mediated Fe-DMA transport by Cd is not related to Cd-DMA complex formation and that Cd-induced phytosiderophore release cannot protect maize plants from Cd toxicity. Instead, phytosiderophore-mediated Fe acquisition can improve Fe uptake in the presence of Cd and thereby provides an advantage under Cd stress relative to Fe acquisition via ferrous Fe.
Figures







References
-
- Awad F, Römheld V (2000) Mobilization of heavy metals from contaminated calcareous soils by plant born, microbial and synthetic chelators and their uptake by wheat plants. J Plant Nutr 23 1847–1855
-
- Cakmak I, Erenoglu B, Gülüt KY, Derici R, Römheld V (1998) Light-mediated release of phytosiderophores in wheat and barley under iron or zinc deficiency. Plant Soil 202 309–315
-
- Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88 1707–1719 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

