Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states
- PMID: 17337582
- PMCID: PMC1913414
- DOI: 10.1128/JB.00107-07
Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states
Abstract
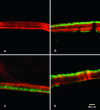
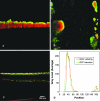
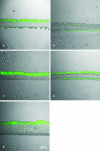
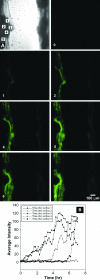
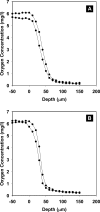
It has long been suspected that microbial biofilms harbor cells in a variety of activity states, but there have been few direct experimental visualizations of this physiological heterogeneity. Spatial patterns of DNA replication and protein synthetic activity were imaged and quantified in staphylococcal biofilms using immunofluorescent detection of pulse-labeled DNA and also an inducible green fluorescent protein (GFP) construct. Stratified patterns of DNA synthetic and protein synthetic activity were observed in all three biofilm systems to which the techniques were applied. In a colony biofilm system, the dimensions of the zone of anabolism at the air interface ranged from 16 to 38 microm and corresponded with the depth of oxygen penetration measured with a microelectrode. A second zone of activity was observed along the nutrient interface of the biofilm. Much of the biofilm was anabolically inactive. Since dead cells constituted only 10% of the biofilm population, most of the inactive cells in the biofilm were still viable. Collectively, these results suggest that staphylococcal biofilms contain cells in at least four distinct states: growing aerobically, growing fermentatively, dead, and dormant. The variety of activity states represented in a biofilm may contribute to the special ecology and tolerance to antimicrobial agents of biofilms.
Figures
References
-
- Andreoli-Pinto, T. J., and K. U. Graziano. 1999. Important aspects of the colonization of central venous catheter. Boll. Chim. Farm. 138:19-23. - PubMed
-
- Artusson, V., R. D. Finlay, and J. K. Jansson. 2005. Combined bromodeoxyuridine immunocapture and terminal-restriction fragment length polymorphism analysis highlights differences in the active soil bacterial metagenome due to Glomus mosseae inoculation or plant species. Environ. Microbiol. 7:1952-1966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

