Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14
- PMID: 17337585
- PMCID: PMC1855903
- DOI: 10.1128/JB.01685-06
Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14
Abstract
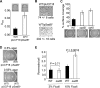
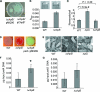
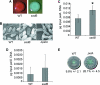
We previously reported that SadB, a protein of unknown function, is required for an early step in biofilm formation by the opportunistic pathogen Pseudomonas aeruginosa. Here we report that a mutation in sadB also results in increased swarming compared to the wild-type strain. Our data are consistent with a model in which SadB inversely regulates biofilm formation and swarming motility via its ability both to modulate flagellar reversals in a viscosity-dependent fashion and to influence the production of the Pel exopolysaccharide. We also show that SadB is required to properly modulate flagellar reversal rates via chemotaxis cluster IV (CheIV cluster). Mutational analyses of two components of the CheIV cluster, the methyl-accepting chemotaxis protein PilJ and the PilJ demethylase ChpB, support a model wherein this chemotaxis cluster participates in the inverse regulation of biofilm formation and swarming motility. Epistasis analysis indicates that SadB functions upstream of the CheIV cluster. We propose that P. aeruginosa utilizes a SadB-dependent, chemotaxis-like regulatory pathway to inversely regulate two key surface behaviors, biofilm formation and swarming motility.
Figures

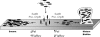
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

