Synthesis of some members of the hydroxylated phenanthridone subclass of the Amaryllidaceae alkaloid family
- PMID: 17338575
- PMCID: PMC2475589
- DOI: 10.1021/jo0626111
Synthesis of some members of the hydroxylated phenanthridone subclass of the Amaryllidaceae alkaloid family
Abstract
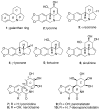
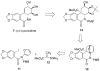
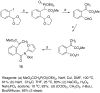
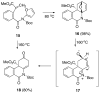
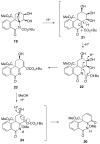
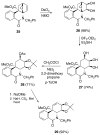
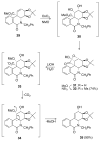
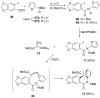
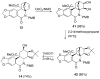
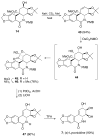
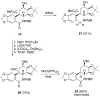
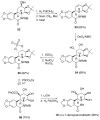
The total synthesis of several members of the hydroxylated phenanthridone subclass of the Amaryllidaceae alkaloid family has been carried out. (+/-)-Lycoricidine and (+/-)-7-deoxypancratistatin were assembled through a one-pot Stille/intramolecular Diels-Alder cycloaddition cascade to construct the core skeleton. The initially formed [4+2]-cycloadduct undergoes nitrogen-assisted ring opening followed by a deprotonation/reprotonation of the resulting zwitterion to give a rearranged hexahydroindolinone on further heating at 160 degrees C. The stereochemical outcome of the IMDAF cycloaddition has the side arm of the tethered vinyl group oriented exo with respect to the oxygen bridge. The resulting cycloadduct was used for the stereocontrolled installation of the remaining functionality present in the C-ring of the target molecules. Key features of the synthetic strategy include (1) a lithium hydroxide induced tandem hydrolysis/decarboxylation/elimination sequence to introduce the required pi-bond in the C-ring of (+/-)-lycoricidine, and (2) conversion of the initially formed Diels-Alder adduct into an aldehyde intermediate which then undergoes a stereospecific decarbonylation reaction mediated by Wilkinson's catalyst to set the trans-B-C ring junction of (+/-)-7-deoxypancratistatin.
Figures
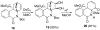
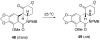
References
-
- Martin SF. The Amaryllidaceae Alkaloids, Chapter 3. In: Brossi A, editor. The Alkaloids. Vol. 30. Academic Press; New York: 1987. p. 251.
-
- Lewis JR. Nat Prod Rep. 1994;11:329. - PubMed
-
- Cook JW, Loudon JD. In: The Alkaloids. Manske RHF, Holmes HL, editors. Vol. 2. Academic Press; New York: 1952. p. 331.
-
- Ghosal S, Saini KS, Razdan S. Phytochem. 1985;24:2141.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

