Adaptive divergence in experimental populations of Pseudomonas fluorescens. III. Mutational origins of wrinkly spreader diversity
- PMID: 17339222
- PMCID: PMC1893022
- DOI: 10.1534/genetics.106.069906
Adaptive divergence in experimental populations of Pseudomonas fluorescens. III. Mutational origins of wrinkly spreader diversity
Abstract
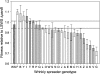
Understanding the connections among genotype, phenotype, and fitness through evolutionary time is a central goal of evolutionary genetics. Wrinkly spreader (WS) genotypes evolve repeatedly in model Pseudomonas populations and show substantial morphological and fitness differences. Previous work identified genes contributing to the evolutionary success of WS, in particular the di-guanylate cyclase response regulator, WspR. Here we scrutinize the Wsp signal transduction pathway of which WspR is the primary output component. The pathway has the hallmarks of a chemosensory pathway and genetic analyses show that regulation and function of Wsp is analogous to the Che chemotaxis pathway from Escherichia coli. Of significance is the methyltransferase (WspC) and methylesterase (WspF) whose opposing activities form an integral feedback loop that controls the activity of the kinase (WspE). Deductions based on the regulatory model suggested that mutations within wspF were a likely cause of WS. Analyses of independent WS genotypes revealed numerous simple mutations in this single open reading frame. Remarkably, different mutations have different phenotypic and fitness effects. We suggest that the negative feedback loop inherent in Wsp regulation allows the pathway to be tuned by mutation in a rheostat-like manner.
Figures

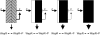
References
-
- Alonso, C. R., and A. S. Wilkins, 2005. Opinion: the molecular elements that underlie developmental evolution. Nat. Rev. Genet. 6: 709–715. - PubMed
-
- Baker, M. D., P. M. Wolanin and J. B. Stock, 2006. Signal transduction in bacterial chemotaxis. BioEssays 28: 9–22. - PubMed
-
- Bantinaki, E., 2002. Characterization of a novel chemosensory pathway underlying adaptive evolution in experimental population of Pseudomonas fluorescens SBW25. Ph.D. Thesis, University of Oxford, Oxford.
-
- Bush, R. M., C. A. Bender, K. Subbarao, N. J. Cox and W. M. Fitch, 1999. Predicting the evolution of human influenza A. Science 286: 1921–1925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

