Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members
- PMID: 17339340
- PMCID: PMC1899957
- DOI: 10.1128/MCB.02249-06
Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members
Abstract
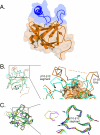
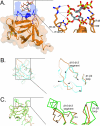
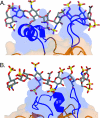
Unique among fibroblast growth factors (FGFs), FGF19, -21, and -23 act in an endocrine fashion to regulate energy, bile acid, glucose, lipid, phosphate, and vitamin D homeostasis. These FGFs require the presence of Klotho/betaKlotho in their target tissues. Here, we present the crystal structures of FGF19 alone and FGF23 in complex with sucrose octasulfate, a disaccharide chemically related to heparin. The conformation of the heparin-binding region between beta strands 10 and 12 in FGF19 and FGF23 diverges completely from the common conformation adopted by paracrine-acting FGFs. A cleft between this region and the beta1-beta2 loop, the other heparin-binding region, precludes direct interaction between heparin/heparan sulfate and backbone atoms of FGF19/23. This reduces the heparin-binding affinity of these ligands and confers endocrine function. Klotho/betaKlotho have evolved as a compensatory mechanism for the poor ability of heparin/heparan sulfate to promote binding of FGF19, -21, and -23 to their cognate receptors.
Figures


References
-
- Araya, K., S. Fukumoto, R. Backenroth, Y. Takeuchi, K. Nakayama, N. Ito, N. Yoshii, Y. Yamazaki, T. Yamashita, J. Silver, T. Igarashi, and T. Fujita. 2005. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J. Clin. Endocrinol Metab. 90:5523-5527. - PubMed
-
- Bottcher, R. T., and C. Niehrs. 2005. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 26:63-77. - PubMed
-
- Harmer, N. J., L. Pellegrini, D. Chirgadze, J. Fernandez-Recio, and T. L. Blundell. 2004. The crystal structure of fibroblast growth factor (FGF) 19 reveals novel features of the FGF family and offers a structural basis for its unusual receptor affinity. Biochemistry 43:629-640. - PubMed
-
- Holt, J. A., G. Luo, A. N. Billin, J. Bisi, Y. Y. McNeill, K. F. Kozarsky, M. Donahee, Y. Wang da, T. A. Mansfield, S. A. Kliewer, B. Goodwin, and S. A. Jones. 2003. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 17:1581-1591. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG25326/AG/NIA NIH HHS/United States
- AG19712/AG/NIA NIH HHS/United States
- DK067158/DK/NIDDK NIH HHS/United States
- R01 AG019712/AG/NIA NIH HHS/United States
- GM38060-17/GM/NIGMS NIH HHS/United States
- DK063934/DK/NIDDK NIH HHS/United States
- R01 HL052622/HL/NHLBI NIH HHS/United States
- S10 RR017990/RR/NCRR NIH HHS/United States
- R01 HL062244/HL/NHLBI NIH HHS/United States
- R01 DK067158/DK/NIDDK NIH HHS/United States
- P30 NS050276/NS/NINDS NIH HHS/United States
- R01 GM038060/GM/NIGMS NIH HHS/United States
- DE13686/DE/NIDCR NIH HHS/United States
- R01 DE013686/DE/NIDCR NIH HHS/United States
- HL62244/HL/NHLBI NIH HHS/United States
- R01 DK063934/DK/NIDDK NIH HHS/United States
- R01 AG025326/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
