Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes
- PMID: 17339344
- PMCID: PMC1899973
- DOI: 10.1128/MCB.01719-06
Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes
Abstract
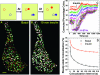
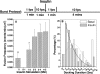
Total internal reflection fluorescence (TIRF) microscopy reveals highly mobile structures containing enhanced green fluorescent protein-tagged glucose transporter 4 (GLUT4) within a zone about 100 nm beneath the plasma membrane of 3T3-L1 adipocytes. We developed a computer program (Fusion Assistant) that enables direct analysis of the docking/fusion kinetics of hundreds of exocytic fusion events. Insulin stimulation increases the fusion frequency of exocytic GLUT4 vesicles by approximately 4-fold, increasing GLUT4 content in the plasma membrane. Remarkably, insulin signaling modulates the kinetics of the fusion process, decreasing the vesicle tethering/docking duration prior to membrane fusion. In contrast, the kinetics of GLUT4 molecules spreading out in the plasma membrane from exocytic fusion sites is unchanged by insulin. As GLUT4 accumulates in the plasma membrane, it is also immobilized in punctate structures on the cell surface. A previous report suggested these structures are exocytic fusion sites (Lizunov et al., J. Cell Biol. 169:481-489, 2005). However, two-color TIRF microscopy using fluorescent proteins fused to clathrin light chain or GLUT4 reveals these structures are clathrin-coated patches. Taken together, these data show that insulin signaling accelerates the transition from docking of GLUT4-containing vesicles to their fusion with the plasma membrane and promotes GLUT4 accumulation in clathrin-based endocytic structures on the plasma membrane.
Figures






References
-
- Bai, L., Y. Wang, J. Fan, Y. Chen, W. Ji, A. Qu, P. Xu, D. E. James, and T. Xu. 2007. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 5:47-57. - PubMed
-
- Bellve, K. D., D. Leonard, C. Standley, L. M. Lifshitz, R. A. Tuft, A. Hayakawa, S. Corvera, and K. E. Fogarty. 2006. Plasma membrane domains specialized for clathrin-mediated endocytosis in primary cells. J. Biol. Chem. 281:16139-16146. - PubMed
-
- Bose, A., A. Guilherme, S. I. Robida, S. M. Nicoloro, Q. L. Zhou, Z. Y. Jiang, D. P. Pomerleau, and M. P. Czech. 2002. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420:821-824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
