Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation
- PMID: 17339346
- PMCID: PMC1865774
- DOI: 10.1128/IAI.00085-07
Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation
Abstract
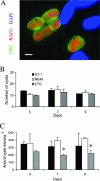
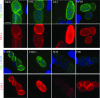
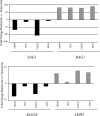
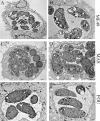
Toxoplasma gondii undergoes differentiation from rapidly growing tachyzoites to slowly growing bradyzoites during its life cycle in the intermediate host, and conversion can be induced in vitro by stress. Representative strains of the three clonal lineages showed equal capacity to differentiate into bradyzoites in vitro, as evidenced by induction of bradyzoite antigen 1, staining with Dolichos biflorus lectin (DBL), pepsin resistance, and oral infectivity in mice. We also examined several recently described exotic strains of T. gondii, which are genetically diverse and have a different ancestry from the clonal lineages. The exotic strain COUG was essentially like the clonal lineages and showed a high capacity to induce bradyzoites in vitro and in vivo, consistent with its ability to be efficiently transmitted by the oral route. In contrast, exotic strains MAS and FOU, which are defective in oral transmission, showed a decreased potential to develop into bradyzoites in vitro. This defect was evident from reduced staining with DBL and the cyst antigen CST1, failure to down-regulate tachyzoite antigens, such as tachyzoite surface antigens 1 and 2A, and decreased resistance to pepsin treatment. Despite normal in vitro differentiation, the exotic strains CAST and GPHT also showed decreased oral transmission, due to formation of smaller cysts and a lower tissue burden during chronic infection, traits also shared by MAS and FOU. Collectively, these findings reveal that the limited oral transmission in some strains of T. gondii is due to inefficient differentiation to the bradyzoite form, leading to defects in the formation of tissue cysts.
Figures

Similar articles
-
Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts.Vet Parasitol. 2006 Aug 31;140(1-2):69-75. doi: 10.1016/j.vetpar.2006.03.018. Epub 2006 Apr 27. Vet Parasitol. 2006. PMID: 16647212
-
Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host.Int J Parasitol. 2004 Mar 9;34(3):347-60. doi: 10.1016/j.ijpara.2003.11.024. Int J Parasitol. 2004. PMID: 15003495
-
Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii.J Eukaryot Microbiol. 1997 Nov-Dec;44(6):592-602. doi: 10.1111/j.1550-7408.1997.tb05965.x. J Eukaryot Microbiol. 1997. PMID: 9435131
-
Advances in the life cycle of Toxoplasma gondii.Int J Parasitol. 1998 Jul;28(7):1019-24. doi: 10.1016/s0020-7519(98)00023-x. Int J Parasitol. 1998. PMID: 9724872 Review.
-
Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts.Clin Microbiol Rev. 1998 Apr;11(2):267-99. doi: 10.1128/CMR.11.2.267. Clin Microbiol Rev. 1998. PMID: 9564564 Free PMC article. Review.
Cited by
-
Starvation of low-density lipoprotein-derived cholesterol induces bradyzoite conversion in Toxoplasma gondii.Parasit Vectors. 2014 May 29;7:248. doi: 10.1186/1756-3305-7-248. Parasit Vectors. 2014. PMID: 24885547 Free PMC article.
-
Mitochondrial metabolism of glucose and glutamine is required for intracellular growth of Toxoplasma gondii.Cell Host Microbe. 2012 Nov 15;12(5):682-92. doi: 10.1016/j.chom.2012.09.013. Cell Host Microbe. 2012. PMID: 23159057 Free PMC article.
-
Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens.Cell Host Microbe. 2008 Nov 13;4(5):458-69. doi: 10.1016/j.chom.2008.10.003. Cell Host Microbe. 2008. PMID: 18996346 Free PMC article.
-
In vitro maturation of Toxoplasma gondii bradyzoites in human myotubes and their metabolomic characterization.Nat Commun. 2022 Mar 4;13(1):1168. doi: 10.1038/s41467-022-28730-w. Nat Commun. 2022. PMID: 35246532 Free PMC article.
-
Succinylated Wheat Germ Agglutinin Colocalizes with the Toxoplasma gondii Cyst Wall Glycoprotein CST1.mSphere. 2020 Mar 4;5(2):e00031-20. doi: 10.1128/mSphere.00031-20. mSphere. 2020. PMID: 32132158 Free PMC article.
References
-
- Ajzenberg, D., A. L. Bañuls, C. Su, A. Dumètre, M. Demar, B. Carme, and M. L. Dardé. 2004. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int. J. Parasitol. 34:1185-1196. - PubMed
-
- Ajzenberg, D., N. Cogné, L. Paris, M. H. Bessieres, P. Thulliez, D. Fillisetti, H. Pelloux, P. Marty, and M. L. Dardé. 2002. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis and correlation with clinical findings. J. Infect. Dis. 186:684-689. - PubMed
-
- Aramini, J., C. Stephen, and J. Dubey. 1998. Toxoplasma gondii in Vancouver Island cougars (Felis concolor vancouverensis): serology and oocyst shedding. J. Parasitol. 84:438-440. - PubMed
-
- Bohne, W., U. Gross, D. J. P. Ferguson, and J. Hessemann. 1995. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol. Microbiol. 16:1221-1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

