The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease
- PMID: 17339406
- PMCID: PMC2137908
- DOI: 10.1084/jem.20061931
The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease
Abstract
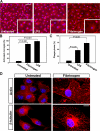
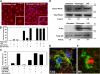
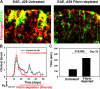
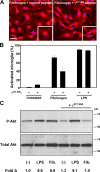
Perivascular microglia activation is a hallmark of inflammatory demyelination in multiple sclerosis (MS), but the mechanisms underlying microglia activation and specific strategies to attenuate their activation remain elusive. Here, we identify fibrinogen as a novel regulator of microglia activation and show that targeting of the interaction of fibrinogen with the microglia integrin receptor Mac-1 (alpha(M)beta(2), CD11b/CD18) is sufficient to suppress experimental autoimmune encephalomyelitis in mice that retain full coagulation function. We show that fibrinogen, which is deposited perivascularly in MS plaques, signals through Mac-1 and induces the differentiation of microglia to phagocytes via activation of Akt and Rho. Genetic disruption of fibrinogen-Mac-1 interaction in fibrinogen-gamma(390-396A) knock-in mice or pharmacologically impeding fibrinogen-Mac-1 interaction through intranasal delivery of a fibrinogen-derived inhibitory peptide (gamma(377-395)) attenuates microglia activation and suppresses relapsing paralysis. Because blocking fibrinogen-Mac-1 interactions affects the proinflammatory but not the procoagulant properties of fibrinogen, targeting the gamma(377-395) fibrinogen epitope could represent a potential therapeutic strategy for MS and other neuroinflammatory diseases associated with blood-brain barrier disruption and microglia activation.
Figures



References
-
- Lassmann, H., W. Bruck, and C. Lucchinetti. 2001. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol. Med. 7:115–121. - PubMed
-
- Platten, M., and L. Steinman. 2005. Multiple sclerosis: trapped in deadly glue. Nat. Med. 11:252–253. - PubMed
-
- Heppner, F.L., M. Greter, D. Marino, J. Falsig, G. Raivich, N. Hovelmeyer, A. Waisman, T. Rulicke, M. Prinz, J. Priller, et al. 2005. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11:146–152. - PubMed
-
- Jack, C., F. Ruffini, A. Bar-Or, and J.P. Antel. 2005. Microglia and multiple sclerosis. J. Neurosci. Res. 81:363–373. - PubMed
-
- Minagar, A., and J.S. Alexander. 2003. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. 9:540–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

