A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1
- PMID: 17339485
- PMCID: PMC2041900
- DOI: 10.4049/jimmunol.178.6.3856
A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1
Abstract
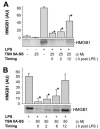
The pathogenesis of sepsis is mediated in part by bacterial endotoxin, which stimulates macrophages/monocytes to sequentially release early (e.g., TNF, IL-1, and IFN-gamma) and late (e.g., high mobility group box 1 (HMGB1) protein) proinflammatory cytokines. The recent discovery of HMGB1 as a late mediator of lethal sepsis has prompted investigation for development of new experimental therapeutics. We found that many steroidal drugs (such as dexamethasone and cortisone) and nonsteroidal anti-inflammatory drugs (such as aspirin, ibuprofen, and indomethacin) failed to influence endotoxin-induced HMGB1 release even at superpharmacological concentrations (up to 10-25 microM). However, several steroid-like pigments (tanshinone I, tanshinone IIA, and cryptotanshinone) of a popular Chinese herb, Danshen (Salvia miltiorrhiza), dose dependently attenuated endotoxin-induced HMGB1 release in macrophage/monocyte cultures. A water-soluble tanshinone IIA sodium sulfonate derivative (TSNIIA-SS), which has been widely used as a Chinese medicine for patients with cardiovascular disorders, selectively abrogated endotoxin-induced HMGB1 cytoplasmic translocation and release in a glucocorticoid receptor-independent manner. Administration of TSNIIA-SS significantly protected mice against lethal endotoxemia and rescued mice from lethal sepsis even when the first dose was given 24 h after the onset of sepsis. The therapeutic effects were partly attributable to attenuation of systemic accumulation of HMGB1 (but not TNF and NO) and improvement of cardiovascular physiologic parameters (e.g., decrease in total peripheral vascular resistance and increase in cardiac stroke volume) in septic animals. Taken together, these data re-enforce the pathogenic role of HMGB1 in lethal sepsis, and support a therapeutic potential for TSNIIA-SS in the treatment of human sepsis.
Figures







References
-
- Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. - PubMed
-
- Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol. Today. 1991;12:404–410. - PubMed
-
- Heinzel FP. The role of IFN-γ in the pathology of experimental endotoxemia. J. Immunol. 1990;145:2920–2924. - PubMed
-
- Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 2000;6:164–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

