VCAM-1 activation of endothelial cell protein tyrosine phosphatase 1B
- PMID: 17339486
- PMCID: PMC2710028
- DOI: 10.4049/jimmunol.178.6.3865
VCAM-1 activation of endothelial cell protein tyrosine phosphatase 1B
Abstract
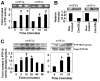
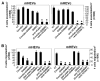
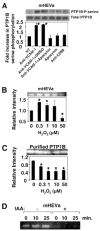
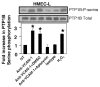
Lymphocytes migrate from the blood into tissue by binding to and migrating across endothelial cells. One of the endothelial cell adhesion molecules that mediate lymphocyte binding is VCAM-1. We have reported that binding to VCAM-1 activates endothelial cell NADPH oxidase for the generation of reactive oxygen species (ROS). The ROS oxidize and stimulate an increase in protein kinase C (PKC)alpha activity. Furthermore, these signals are required for VCAM-1-dependent lymphocyte migration. In this report, we identify a role for protein tyrosine phosphatase 1B (PTP1B) in the VCAM-1 signaling pathway. In primary cultures of endothelial cells and endothelial cell lines, Ab cross-linking of VCAM-1 stimulated an increase in serine phosphorylation of PTP1B, the active form of PTP1B. Ab cross-linking of VCAM-1 also increased activity of PTP1B. This activation of PTP1B was downstream of NADPH oxidase and PKCalpha in the VCAM-1 signaling pathway as determined with pharmacological inhibitors and antisense approaches. In addition, during VCAM-1 signaling, ROS did not oxidize endothelial cell PTP1B. Instead PTP1B was activated by serine phosphorylation. Importantly, inhibition of PTP1B activity blocked VCAM-1-dependent lymphocyte migration across endothelial cells. In summary, VCAM-1 activates endothelial cell NADPH oxidase to generate ROS, resulting in oxidative activation of PKCalpha and then serine phosphorylation of PTP1B. This PTP1B activity is necessary for VCAM-1-dependent transendothelial lymphocyte migration. These data show, for the first time, a function for PTP1B in VCAM-1-dependent lymphocyte migration.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures

References
-
- Chin JE, Hatfield CA, Winterrowd GE, Brashler JR, Vonderfecht SL, Fidler SF, Griffin RL, Kolbasa KP, Krzesicki RF, Sly LM, et al. Airway recruitment of leukocytes in mice is dependent on α4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–L229. - PubMed
-
- Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: a potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100:1223–1229. - PubMed
-
- Wright PS, Cooper JR, Kropp KE, Busch SJ. Induction of vascular cell adhesion molecule-1 expression by IL-4 in human aortic smooth muscle cells is not associated with increased nuclear NF-κB levels. J Cell Physiol. 1999;180:381–389. - PubMed
-
- Balogh P, Aydar Y, Tew JG, Szakal AK. Appearance and phenotype of murine follicular dendritic cells expressing VCAM-1. Anat Rec. 2002;268:160–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

