Methyl succinate antagonises biguanide-induced AMPK-activation and death of pancreatic beta-cells through restoration of mitochondrial electron transfer
- PMID: 17339833
- PMCID: PMC2013909
- DOI: 10.1038/sj.bjp.0707189
Methyl succinate antagonises biguanide-induced AMPK-activation and death of pancreatic beta-cells through restoration of mitochondrial electron transfer
Abstract
Background and purpose: Two mechanisms have been proposed to explain the insulin-sensitising properties of metformin in peripheral tissues: (a) inhibition of electron transport chain complex I, and (b) activation of the AMP activated protein kinase (AMPK). However the relationship between these mechanisms and their contribution to beta-cell death and dysfunction in vitro, are currently unclear.
Experimental approach: The effects of biguanides (metformin and phenformin) were tested on MIN6 beta-cells and primary FACS-purified rat beta-cells. Cell metabolism was assessed biochemically and by FACS analysis, and correlated with AMPK phosphorylation state and cell viability, with or without fuel substrates.
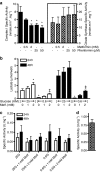
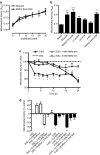
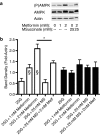
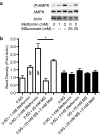
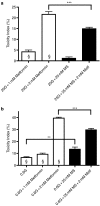
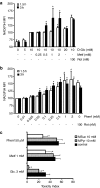
Key results: In MIN6 cells, metformin reduced mitochondrial complex I activity by up to 44% and a 25% net reduction in mitochondrial reducing potential. In rat beta-cells, metformin caused NAD(P)H accumulation above maximal glucose-inducible levels, mimicking the effect of rotenone. Drug exposure caused phosphorylation of AMPK on Thr(172) in MIN6 cell extracts, indicative of kinase activation. Methyl succinate, a complex II substrate, appeared to bypass metformin blockade of complex I. This resulted in reduced phosphorylation of AMPK, establishing a link between biguanide-induced mitochondrial inhibition and AMPK activation. Corresponding assessment of cell death indicated that methyl succinate decreased biguanide toxicity to beta-cells in vitro.
Conclusions and implications: AMPK activation can partly be attributed to metformin's inhibitory action on mitochondrial complex I. Anaplerotic fuel metabolism via complex II rescued beta-cells from metformin-associated toxicity. We propose that utilisation of anaplerotic nutrients may reconcile in vitro and in vivo effects of metformin on the pancreatic beta-cell.
Figures
References
-
- Abbud W, Habinowski S, Zhang JZ, Kendrew J, Elkairi FS, Kemp BE, et al. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch Biochem Biophys. 2000;380:347–352. - PubMed
-
- Abramson E, Arky RA. Treatment of the obese diabetic. A comparative study of placebo, sulfonylurea and phenformin. Metabolism. 1967;16:204–212. - PubMed
-
- Ainscow EK, Zhao C, Rutter GA. Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion. Diabetes. 2000;49:1149–1155. - PubMed
-
- Bennett BD, Jetton TL, Ying G, Magnuson MA, Piston DW. Quantitative subcellular imaging of glucose metabolism within intact pancreatic islets. J Biol Chem. 1996;271:3647–3651. - PubMed
-
- Bergmeyer H, Bernt E.Lactate dehydrogenase: UV-assay with pyruvate and NADH Methods of Enzymatic Analysis 1974Verlag Chemie Weinheim, Academic Press, Inc.: New York; 574–579.In: Bergmeyer H (ed)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

