The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells
- PMID: 17339836
- PMCID: PMC2013913
- DOI: 10.1038/sj.bjp.0707184
The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells
Abstract
Background and purpose: Recent evidence indicates that carbon monoxide-releasing molecules (CO-RMs) exhibit potential anti-inflammatory properties. In the present study, we have investigated whether tricarbonyl dichloro ruthenium(II) dimer (CORM-2) can control the inflammatory response induced by cytokines in a human colonic epithelial cell line, Caco-2.
Experimental approach: Caco-2 cells were preincubated with CORM-2 for 30 minutes and then stimulated with interleukin (IL)-1beta, tumor necrosis factor-alpha and interferon-gamma for different times. Gene expression was analyzed by real-time PCR. Protein expression was investigated by Western blot and ELISA. Transcription factor activation was determined by the luciferase method.
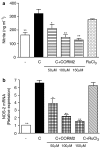
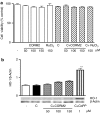
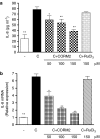
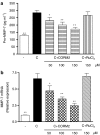
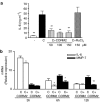
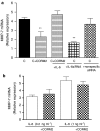
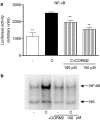
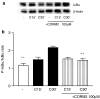
Key results: We have shown that CORM-2 significantly decreased the mRNA expression of nitric oxide synthase-2 (NOS-2) and the production of nitrite, in Caco-2 cells stimulated with cytokines. IL-8, IL-6 and metalloproteinase-7 (MMP-7) mRNA and protein were also significantly reduced by CORM-2. Time-course and small interfering RNA studies suggest that inhibition of IL-6 plays a role in the regulation of MMP-7 expression by CORM-2. These effects of CORM-2 can be dependent on the modulation of nuclear factor-kappaB (NF-kappaB), activator protein-1, CCAT/enhancer binding protein and the phosphorylated forms of NF-kappaB inhibitory protein-alpha, c-Jun N-terminal protein kinase 1/2, p38 and extracellular signal-regulated kinase 1/2.
Conclusions and implications: CORM-2 can regulate a number of genes relevant in intestinal inflammation and cancer progression. These findings provide new insights into the anti-inflammatory properties and potential applications of this class of compounds.
Figures


Comment in
-
Physiological activities of carbon monoxide-releasing molecules: Ca ira.Br J Pharmacol. 2007 Apr;150(8):961-2. doi: 10.1038/sj.bjp.0707185. Epub 2007 Mar 5. Br J Pharmacol. 2007. PMID: 17339835 Free PMC article.
References
-
- Ambs S, Merriam WG, Ogunfusika MO, Bennett WP, Ishibe N, Hussain SP, et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med. 1998;4:1371–1376. - PubMed
-
- Bani-Hani MG, Greenstein D, Mann BE, Green CJ, Motterlini R. Modulation of thrombin-induced neuroinflammation in BV-2 microglia by carbon monoxide-releasing molecule 3. J Pharmacol Exp Ther. 2006;318:1315–1322. - PubMed
-
- Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–822. - PubMed
-
- Berberat PO, Rahim YI, Yamashita K, Warny MM, Csizmadia E, Robson SC, et al. Heme oxygenase-1-generated biliverdin ameliorates experimental murine colitis. Inflamm Bowel Dis. 2005;11:350–359. - PubMed
-
- Bister V, Salmela MT, Heikkila P, Anttila A, Rintala R, Isaka K, et al. Matrilysins-1 and -2 (MMP-7 and -26) and metalloelastase (MMP-12), unlike MMP-19, are up-regulated in necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2005;40:60–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

