Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity
- PMID: 17339844
- PMCID: PMC1952181
- DOI: 10.1038/sj.bjp.0707161
Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity
Abstract
Background and purpose: Clinical indications for erythropoietin (EPO) in the vascular system reach far beyond the treatment of anemia, but the development of EPO as a non-toxic agent rests heavily upon the cellular pathways controlled by EPO that require elucidation.
Experimental approach: We modulated gene activity and examined cellular trafficking of critical pathways during oxidative stress that may work in concert with EPO to protect primary cerebral endothelial cells (ECs) during oxidative stress, namely protein kinase B (Akt1), 14-3-3 protein, the Forkhead transcription factor FOXO3a.
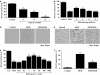
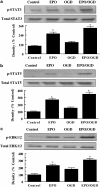
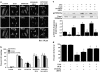
Key results: Here, we show that preservation of ECs by EPO during oxygen-glucose deprivation (OGD) required the initial activation of the phosphatidylinositol 3-kinase (PI-3K) pathway through Akt1, since specific pharmacological blockade of Akt1 activity or gene silencing of Akt1 prevented EC protection by EPO. EPO subsequently involved a series of anti-apoptotic pathways to activate STAT3, STAT5, and ERK 1/2. Furthermore, EPO maintained the inhibitory phosphorylation and integrity of the 'pro-apoptotic' transcription factor FOXO3a, promoted the binding of FOXO3a to 14-3-3 protein and regulated the intracellular trafficking of FOXO3a. Additionally, gene silencing of FOXO3a during OGD significantly increased EC survival, but did not synergistically improve cytoprotection by EPO, illustrating that EPO relied upon the blockade of the FOXO3a pathway.
Conclusions and implications: Our work defines a novel cytoprotective pathway in ECs that involves PI-3 K, STAT3, STAT5, ERK 1/2, 14-3-3 protein and FOXO3a, which can be targeted for the development of EPO as a clinically effective and safe agent in the vascular system.
Figures




Comment in
-
EPO tecting the endothelium.Br J Pharmacol. 2007 Apr;150(7):823-5. doi: 10.1038/sj.bjp.0707162. Epub 2007 Mar 12. Br J Pharmacol. 2007. PMID: 17351666 Free PMC article.
References
-
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood–brain barrier. J Cell Sci. 1992;103:23–37. - PubMed
-
- Avasarala JR, Konduru SS. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-alpha-treated primary cultures of human microvascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25:183–189. - PubMed
-
- Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, et al. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–1012. - PubMed
-
- Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and naurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:e635–e640. - PubMed
-
- Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

