Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons
- PMID: 17341134
- PMCID: PMC1808488
- DOI: 10.1371/journal.pbio.0050063
Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons
Abstract
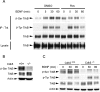
Neurotrophins are key regulators of neuronal survival and differentiation during development. Activation of their cognate receptors, Trk receptors, a family of receptor tyrosine kinases (RTKs), is pivotal for mediating the downstream functions of neurotrophins. Recent studies reveal that cyclin-dependent kinase 5 (Cdk5), a serine/threonine kinase, may modulate RTK signaling through phosphorylation of the receptor. Given the abundant expression of both Cdk5 and Trk receptors in the nervous system, and their mutual involvement in the regulation of neuronal architecture and synaptic functions, it is of interest to investigate if Cdk5 may also modulate Trk signaling. In the current study, we report the identification of TrkB as a Cdk5 substrate. Cdk5 phosphorylates TrkB at Ser478 at the intracellular juxtamembrane region of TrkB. Interestingly, attenuation of Cdk5 activity or overexpression of a TrkB mutant lacking the Cdk5 phosphorylation site essentially abolishes brain-derived neurotrophic factor (BDNF)-triggered dendritic growth in primary hippocampal neurons. In addition, we found that Cdk5 is involved in BDNF-induced activation of Rho GTPase Cdc42, which is essential for BDNF-triggered dendritic growth. Our observations therefore reveal an unanticipated role of Cdk5 in TrkB-mediated regulation of dendritic growth through modulation of BDNF-induced Cdc42 activation.
Conflict of interest statement
Figures





References
-
- Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. - PubMed
-
- Segal RA. Selectivity in neurotrophin signaling: Theme and variations. Annu Rev Neurosci. 2003;26:299–330. - PubMed
-
- Fu AK, Fu WY, Cheung J, Tsim KW, Ip FC, et al. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat Neurosci. 2001;4:374–381. - PubMed
-
- Li BS, Ma W, Jaffe H, Zheng Y, Takahashi S, et al. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J Biol Chem. 2003;278:35702–35709. - PubMed
-
- Cheung ZH, Fu AK, Ip NY. Synaptic roles of Cdk5: Implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

