PGC-1alpha/beta upregulation is associated with improved oxidative phosphorylation in cells harboring nonsense mtDNA mutations
- PMID: 17341490
- PMCID: PMC2652746
- DOI: 10.1093/hmg/ddm045
PGC-1alpha/beta upregulation is associated with improved oxidative phosphorylation in cells harboring nonsense mtDNA mutations
Abstract
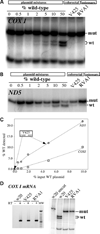
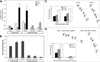
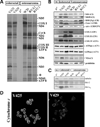
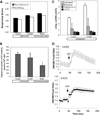
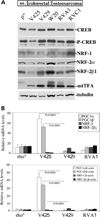
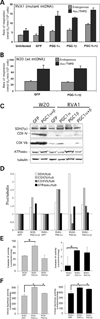
We have studied the functional effects of nonsense mitochondrial DNA (mtDNA) mutations in the COXI and ND5 genes in a colorectal tumor cell line. Surprisingly, these cells had an efficient oxidative phosphorylation (OXPHOS); however, when mitochondria from these cells were transferred to an osteosarcoma nuclear background (osteosarcoma cybrids), the rate of respiration markedly declined suggesting that the phenotypic expression of the mtDNA mutations was prevented by the colorectal tumor nuclear background. We found that there was a significant increase in the steady-state levels of PGC-1alpha and PGC-1beta transcriptional coactivators in these cells and a parallel increase in the steady-state levels of several mitochondrial proteins. Accordingly, adenoviral-mediated overexpression of PGC-1alpha and PGC-1beta in the osteosarcoma cybrids stimulated mitochondrial respiration suggesting that an upregulation of PGC-1alpha/beta coactivators can partially rescue an OXPHOS defect. In conclusion, upregulation of PGC-1alpha and PGC-1beta in the colorectal tumor cells can be part of an adaptation mechanism to help overcome the severe consequences of mtDNA mutations on OXPHOS.
Conflict of interest statement
Figures

Similar articles
-
PGC-1alpha/beta induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders.Hum Mol Genet. 2009 May 15;18(10):1805-12. doi: 10.1093/hmg/ddp093. Epub 2009 Mar 18. Hum Mol Genet. 2009. PMID: 19297390 Free PMC article.
-
An unexpected role for the transcriptional coactivator isoform NT-PGC-1α in the regulation of mitochondrial respiration in brown adipocytes.J Biol Chem. 2017 Jun 16;292(24):9958-9966. doi: 10.1074/jbc.M117.778373. Epub 2017 May 4. J Biol Chem. 2017. PMID: 28473468 Free PMC article.
-
Activation of TGR5 promotes mitochondrial biogenesis in human aortic endothelial cells.Biochem Biophys Res Commun. 2018 Jun 12;500(4):952-957. doi: 10.1016/j.bbrc.2018.04.210. Epub 2018 May 2. Biochem Biophys Res Commun. 2018. PMID: 29709472
-
PGC-1 proteins and heart failure.Trends Cardiovasc Med. 2012 May;22(4):98-105. doi: 10.1016/j.tcm.2012.07.003. Epub 2012 Aug 29. Trends Cardiovasc Med. 2012. PMID: 22939990 Free PMC article. Review.
-
PGC-1α: a master gene that is hard to master.Cell Mol Life Sci. 2012 Aug;69(15):2465-8. doi: 10.1007/s00018-012-1043-0. Epub 2012 Jun 9. Cell Mol Life Sci. 2012. PMID: 22678664 Free PMC article. Review.
Cited by
-
Suppression mechanisms of COX assembly defects in yeast and human: insights into the COX assembly process.Biochim Biophys Acta. 2009 Jan;1793(1):97-107. doi: 10.1016/j.bbamcr.2008.05.003. Epub 2008 May 15. Biochim Biophys Acta. 2009. PMID: 18522805 Free PMC article. Review.
-
Alterations in PGC1α expression levels are involved in colorectal cancer risk: a qualitative systematic review.BMC Cancer. 2017 Nov 9;17(1):731. doi: 10.1186/s12885-017-3725-3. BMC Cancer. 2017. PMID: 29121859 Free PMC article.
-
Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders.Clin Transl Med. 2016 Dec;5(1):25. doi: 10.1186/s40169-016-0104-7. Epub 2016 Jul 27. Clin Transl Med. 2016. PMID: 27465020 Free PMC article. Review.
-
Sequence homology at the breakpoint and clinical phenotype of mitochondrial DNA deletion syndromes.PLoS One. 2010 Dec 20;5(12):e15687. doi: 10.1371/journal.pone.0015687. PLoS One. 2010. PMID: 21187929 Free PMC article.
-
Respiratory Complex I dysfunction in cancer: from a maze of cellular adaptive responses to potential therapeutic strategies.FEBS J. 2022 Dec;289(24):8003-8019. doi: 10.1111/febs.16218. Epub 2021 Oct 18. FEBS J. 2022. PMID: 34606156 Free PMC article. Review.

