Catalytic mechanism of Zn2+-dependent polyol dehydrogenases: kinetic comparison of sheep liver sorbitol dehydrogenase with wild-type and Glu154-->Cys forms of yeast xylitol dehydrogenase
- PMID: 17343568
- PMCID: PMC1896283
- DOI: 10.1042/BJ20061384
Catalytic mechanism of Zn2+-dependent polyol dehydrogenases: kinetic comparison of sheep liver sorbitol dehydrogenase with wild-type and Glu154-->Cys forms of yeast xylitol dehydrogenase
Abstract
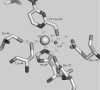
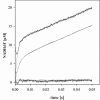
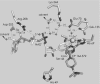
Co-ordination of catalytic Zn2+ in sorbitol/xylitol dehydrogenases of the medium-chain dehydrogenase/reductase superfamily involves direct or water-mediated interactions from a glutamic acid residue, which substitutes a homologous cysteine ligand in alcohol dehydrogenases of the yeast and liver type. Glu154 of xylitol dehydrogenase from the yeast Galactocandida mastotermitis (termed GmXDH) was mutated to a cysteine residue (E154C) to revert this replacement. In spite of their variable Zn2+ content (0.10-0.40 atom/subunit), purified preparations of E154C exhibited a constant catalytic Zn2+ centre activity (kcat) of 1.19+/-0.03 s(-1) and did not require exogenous Zn2+ for activity or stability. E154C retained 0.019+/-0.003% and 0.74+/-0.03% of wild-type catalytic efficiency (kcat/K(sorbitol)=7800+/-700 M(-1) x s(-1)) and kcat (=161+/-4 s(-1)) for NAD+-dependent oxidation of sorbitol at 25 degrees C respectively. The pH profile of kcat/K(sorbitol) for E154C decreased below an apparent pK of 9.1+/-0.3, reflecting a shift in pK by about +1.7-1.9 pH units compared with the corresponding pH profiles for GmXDH and sheep liver sorbitol dehydrogenase (termed slSDH). The difference in pK for profiles determined in 1H2O and 2H2O solvent was similar and unusually small for all three enzymes (approximately +0.2 log units), suggesting that the observed pK in the binary enzyme-NAD+ complexes could be due to Zn2+-bound water. Under conditions eliminating their different pH-dependences, wild-type and mutant GmXDH displayed similar primary and solvent deuterium kinetic isotope effects of 1.7+/-0.2 (E154C, 1.7+/-0.1) and 1.9+/-0.3 (E154C, 2.4+/-0.2) on kcat/K(sorbitol) respectively. Transient kinetic studies of NAD+ reduction and proton release during sorbitol oxidation by slSDH at pH 8.2 show that two protons are lost with a rate constant of 687+/-12 s(-1) in the pre-steady state, which features a turnover of 0.9+/-0.1 enzyme equivalents as NADH was produced with a rate constant of 409+/-3 s(-1). The results support an auxiliary participation of Glu154 in catalysis, and possible mechanisms of proton transfer in sorbitol/xylitol dehydrogenases are discussed.
Figures


References
-
- Riveros-Rosas H., Julian-Sanchez A., Villalobos-Molina R., Pardo J. P., Pina E. Diversity, taxonomy and evolution of medium-chain dehydrogenase/reductase superfamily. Eur. J. Biochem. 2003;270:3309–3334. - PubMed
-
- Persson B., Zigler J. S., Jr, Jörnvall H. A super-family of medium-chain dehydrogenases/reductases (MDR). Sub-lines including ζ-crystallin, alcohol and polyol dehydrogenases, quinone oxidoreductase enoyl reductases, VAT-1 and other proteins. Eur. J. Biochem. 1994;226:15–22. - PubMed
-
- Jeffery J., Jörnvall H. Sorbitol dehydrogenase. Adv. Enzymol. Relat. Areas Mol. Biol. 1988;61:47–106. - PubMed
-
- Eklund H., Horjales E., Jörnvall H., Bränden C. I., Jeffery J. Molecular aspects of functional differences between alcohol and sorbitol dehydrogenases. Biochemistry. 1985;24:8005–8012. - PubMed
-
- Pauly T. A., Ekstrom J. L., Beebe D. A., Chrunyk B., Cunningham D., Griffor M., Kamath A., Lee S. E., Madura R., McGuire D., et al. X-ray crystallographic and kinetic studies of human sorbitol dehydrogenase. Structure. 2003;11:1071–1085. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

