Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts
- PMID: 17343616
- PMCID: PMC2265899
- DOI: 10.1111/j.1365-2567.2006.02538.x
Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts
Abstract
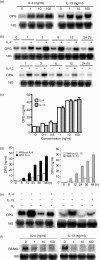
Interleukin (IL)-4 and IL-13 are closely related cytokines known to inhibit osteoclast formation by targeting osteoblasts to produce an inhibitor, osteoprotegerin (OPG), as well as by directly targeting osteoclast precursors. However, whether their inhibitory actions are the same remains unclear. The inhibitory effect of IL-4 was stronger than that of IL-13 in an osteoclast-differentiation culture system containing mouse osteoblasts and osteoclast precursors. Both cytokines induced OPG production by osteoblasts in similar time- and dose-dependent manners. However, IL-4 was stronger in direct inhibition that targeted osteoclast precursors. Furthermore, IL-4 induced phosphorylation of signal transducer and activator of transcription-6 (STAT6) at lower concentrations than those of IL-13 in osteoclast precursors. IL-4 but not IL-13 strongly inhibited the expression of nuclear factor of activated T-cells, cytoplasmic 1 (nuclear factor-ATc1), a key factor of osteoclast differentiation, by those precursors. Thus, the activities of IL-4 and IL-13 toward osteoclast precursors were shown to be different in regards to inhibition of osteoclast differentiation, whereas those toward osteoblasts for inducing OPG expression were equivalent.
Figures





References
-
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–57. - PubMed
-
- Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. - PubMed
-
- Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–42. - PubMed
-
- Yasuda H, Shima N, Nakagawa N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

