Influenza virus infection causes specific degradation of the largest subunit of cellular RNA polymerase II
- PMID: 17344288
- PMCID: PMC1900203
- DOI: 10.1128/JVI.02129-06
Influenza virus infection causes specific degradation of the largest subunit of cellular RNA polymerase II
Erratum in
- J Virol. 2008 Apr;82(7):3811
Abstract
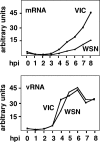
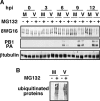
It has been described that influenza virus polymerase associates with RNA polymerase II (RNAP II). To gain information about the role of this interaction, we explored if changes in RNAP II occur during infection. Here we show that influenza virus causes the specific degradation of the hypophosphorylated form of the largest subunit of RNAP II without affecting the accumulation of its hyperphosphorylated forms. This effect is independent of the viral strain and the origin of the cells used. Analysis of synthesized mRNAs in isolated nuclei of infected cells indicated that transcription decreases concomitantly with RNAP II degradation. Moreover, this degradation correlated with the onset of viral transcription and replication. The ubiquitin-mediated proteasome pathway is not involved in virally induced RNAP II proteolysis. The expression of viral polymerase from its cloned cDNAs was sufficient to cause the degradation. Since the PA polymerase subunit has proteolytic activity, we tested its participation in the process. A recombinant virus that encodes a PA point mutant with decreased proteolytic activity and that has defects in replication delayed the effect, suggesting that PA's contribution to RNAP II degradation occurs during infection.
Figures



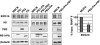

References
-
- Bark-Jones, S. J., H. M. Webb, and M. J. West. 2006. EBV EBNA 2 stimulates CDK9-dependent transcription and RNA polymerase II phosphorylation on serine 5. Oncogene 25:1775-1785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

