Identification of determinants involved in initiation of hepatitis C virus RNA synthesis by using intergenotypic replicase chimeras
- PMID: 17344294
- PMCID: PMC1900214
- DOI: 10.1128/JVI.00032-07
Identification of determinants involved in initiation of hepatitis C virus RNA synthesis by using intergenotypic replicase chimeras
Abstract
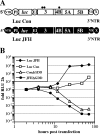
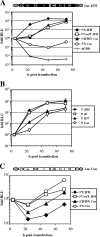
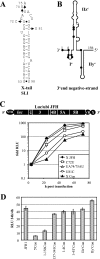
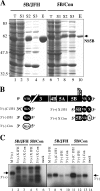
The 5' nontranslated region (NTR) and the X tail in the 3' NTR are the least variable parts of the hepatitis C virus (HCV) genome and play an important role in the initiation of RNA synthesis. By using subgenomic replicons of the HCV isolates Con1 (genotype 1) and JFH1 (genotype 2), we characterized the genotype specificities of the replication signals contained in the NTRs. The replacement of the JFH1 5' NTR and X tail with the corresponding Con1 sequence resulted in a significant decrease in replication efficiency. Exchange of the X tail specifically reduced negative-strand synthesis, whereas substitution of the 5' NTR impaired the generation of progeny positive strands. In search for the proteins involved in the recognition of genotype-specific initiation signals, we analyzed recombinant nonstructural protein 5B (NS5B) RNA polymerases of both isolates and found some genotype-specific template preference for the 3' end of positive-strand RNA in vitro. To further address genotype specificity, we constructed a series of intergenotypic replicon chimeras. When combining NS3 to NS5A of Con1 with NS5B of JFH1, we observed more-efficient replication with the genotype 2a X tail, indicating that NS5B recognizes genotype-specific signals in this region. In contrast, a combination of the NS3 helicase with NS5A and NS5B was required to confer genotype specificity to the 5' NTR. These results present the first genetic evidence for an interaction between helicase, NS5A, and NS5B required for the initiation of RNA synthesis and provide a system for the specific analysis of HCV positive- and negative-strand syntheses.
Figures



References
-
- Bartenschlager, R., M. Frese, and T. Pietschmann. 2004. Novel insights into hepatitis C virus replication and persistence. Adv. Virus Res. 63:71-180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

