Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release
- PMID: 17344385
- PMCID: PMC6672497
- DOI: 10.1523/JNEUROSCI.2279-06.2007
Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release
Abstract
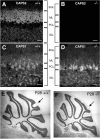
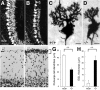
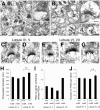
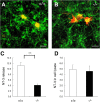
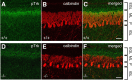
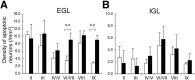
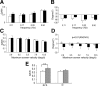
Ca2+-dependent activator protein for secretion 2 (CAPS2/CADPS2) is a secretory granule-associated protein that is abundant at the parallel fiber terminals of granule cells in the mouse cerebellum and is involved in the release of neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF), both of which are required for cerebellar development. The human homolog gene on chromosome 7 is located within susceptibility locus 1 of autism, a disease characterized by several cerebellar morphological abnormalities. Here we report that CAPS2 knock-out mice are deficient in the release of NT-3 and BDNF, and they consequently exhibit suppressed phosphorylation of Trk receptors in the cerebellum; these mice exhibit pronounced impairments in cerebellar development and functions, including neuronal survival, differentiation and migration of postmitotic granule cells, dendritogenesis of Purkinje cells, lobulation between lobules VI and VII, structure and vesicular distribution of parallel fiber-Purkinje cell synapses, paired-pulse facilitation at parallel fiber-Purkinje cell synapses, rotarod motor coordination, and eye movement plasticity in optokinetic training. Increased granule cell death of the external granular layer was noted in lobules VI-VII and IX, in which high BDNF and NT-3 levels are specifically localized during cerebellar development. Therefore, the deficiency of CAPS2 indicates that CAPS2-mediated neurotrophin release is indispensable for normal cerebellar development and functions, including neuronal differentiation and survival, morphogenesis, synaptic function, and motor learning/control. The possible involvement of the CAPS2 gene in the cerebellar deficits of autistic patients is discussed.
Figures


References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994.
-
- Bates B, Rios M, Trumpp A, Chen C, Fan G, Bishop JM, Jaenisch R. Neurotrophin-3 is required for proper cerebellar development. Nat Neurosci. 1999;2:115–117. - PubMed
-
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. - PubMed
-
- Berwin B, Floor E, Martin TF. CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron. 1998;21:137–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
