Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells
- PMID: 17344400
- PMCID: PMC6672502
- DOI: 10.1523/JNEUROSCI.5053-06.2007
Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells
Abstract
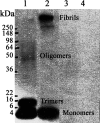
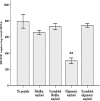
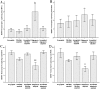
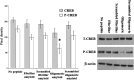
Alzheimer's disease (AD) is a senile dementia characterized by amyloid plaques, neurofibrillary tangles, and synaptic and cell loss. The "amyloid cascade" hypothesis suggests that amyloid-beta (Abeta), the peptide deposited as amyloid plaques, is the primary insult in AD. However, debate continues over the mechanism of Abeta toxicity and whether fibrillar or oligomeric Abeta is the active species of the peptide that ultimately causes the synaptic loss and dementia associated with AD. Brain-derived neurotrophic factor (BDNF) is required for survival and function of cells compromised in AD. Decreased BDNF causes defects in long-term potentiation and memory and correlates with cognitive decline. We previously demonstrated that BDNF reduction occurs early in the course of AD, suggesting that decreased BDNF may promote neuronal dysfunction in AD. We also demonstrated that three of seven human BDNF transcripts are specifically downregulated in AD. What pathological feature(s) of AD leads to the decreased BDNF is unknown. In this study, we administered both fibrillar and oligomeric conformations of Abeta(1-42) to differentiated SH-SY5Y, a human neuroblastoma cell line, and measured both phosphorylated cAMP response element-binding protein (CREB), a regulator of BDNF transcription, and BDNF total mRNA. We found that oligomeric but not fibrillar preparations of Abeta(1-42) significantly decrease both phosphorylated CREB and total BDNF mRNA. Furthermore, oligomeric Abeta(1-42) decreases BDNF transcripts IV and V in these cells, demonstrating that Abeta(1-42) downregulates the major BDNF transcript decreased in vivo in the AD brain. Thus, oligomeric Abeta(1-42) could compromise neuronal function, causing memory loss and cognitive dysfunction by downregulation of BDNF in AD.
Figures

Similar articles
-
CREB expression mediates amyloid β-induced basal BDNF downregulation.Neurobiol Aging. 2015 Aug;36(8):2406-13. doi: 10.1016/j.neurobiolaging.2015.04.014. Epub 2015 Apr 30. Neurobiol Aging. 2015. PMID: 26025137
-
Treatment with the neurotoxic Aβ (25-35) peptide modulates the expression of neuroprotective factors Pin1, Sirtuin 1, and brain-derived neurotrophic factor in SH-SY5Y human neuroblastoma cells.Exp Toxicol Pathol. 2016 May;68(5):271-6. doi: 10.1016/j.etp.2016.02.001. Epub 2016 Feb 22. Exp Toxicol Pathol. 2016. PMID: 26915812
-
Beta -amyloid-(1-42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival Is not compromised.J Biol Chem. 2001 May 18;276(20):17301-6. doi: 10.1074/jbc.M010450200. Epub 2001 Feb 26. J Biol Chem. 2001. PMID: 11278679
-
The role of CREB and BDNF in neurobiology and treatment of Alzheimer's disease.Life Sci. 2020 Sep 15;257:118020. doi: 10.1016/j.lfs.2020.118020. Epub 2020 Jun 27. Life Sci. 2020. PMID: 32603820 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
β-Amyloid (Aβ) oligomers impair brain-derived neurotrophic factor retrograde trafficking by down-regulating ubiquitin C-terminal hydrolase, UCH-L1.J Biol Chem. 2013 Jun 7;288(23):16937-16948. doi: 10.1074/jbc.M113.463711. Epub 2013 Apr 18. J Biol Chem. 2013. PMID: 23599427 Free PMC article.
-
The Role of microRNAs in Alzheimer's Disease and Their Therapeutic Potentials.Genes (Basel). 2018 Mar 21;9(4):174. doi: 10.3390/genes9040174. Genes (Basel). 2018. PMID: 29561798 Free PMC article. Review.
-
Delta-secretase-cleaved Tau antagonizes TrkB neurotrophic signalings, mediating Alzheimer's disease pathologies.Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):9094-9102. doi: 10.1073/pnas.1901348116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004063 Free PMC article.
-
Vitamin D3 administration prevents memory deficit and alteration of biochemical parameters induced by unpredictable chronic mild stress in rats.Sci Rep. 2021 Aug 11;11(1):16271. doi: 10.1038/s41598-021-95850-6. Sci Rep. 2021. PMID: 34381124 Free PMC article.
-
Doublecortin induces mitotic microtubule catastrophe and inhibits glioma cell invasion.J Neurochem. 2009 Jan;108(1):231-45. doi: 10.1111/j.1471-4159.2008.05758.x. J Neurochem. 2009. PMID: 19094064 Free PMC article.
References
-
- Aoyama M, Asai K, Shishikura T, Kawamoto T, Miyachi T, Yokoi T, Togari H, Wada Y, Kato T, Nakagawara A. Human neuroblastomas with unfavorable biologies express high levels of brain-derived neurotrophic factor mRNA and a variety of its variants. Cancer Lett. 2001;164:51–60. - PubMed
-
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. - PubMed
-
- Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, Takahashi K, Gallyas F, Tabira T. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med. 1999;5:560–564. - PubMed
-
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer's disease. Neurobiol Aging. 2003;24:1023–1027. - PubMed
-
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res Mol Brain Res. 1997;49:71–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
