Cellular basis for contrast gain control over the receptive field center of mammalian retinal ganglion cells
- PMID: 17344401
- PMCID: PMC6672510
- DOI: 10.1523/JNEUROSCI.4610-06.2007
Cellular basis for contrast gain control over the receptive field center of mammalian retinal ganglion cells
Abstract
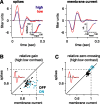
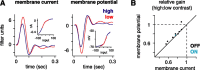
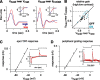
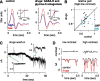
Retinal ganglion cells fire spikes to an appropriate contrast presented over their receptive field center. These center responses undergo dynamic changes in sensitivity depending on the ongoing level of contrast, a process known as "contrast gain control." Extracellular recordings suggested that gain control is driven by a single wide-field mechanism, extending across the center and beyond, that depends on inhibitory interneurons: amacrine cells. However, recordings in salamander suggested that the excitatory bipolar cells, which drive the center, may themselves show gain control independently of amacrine cell mechanisms. Here, we tested in mammalian ganglion cells whether amacrine cells are critical for gain control over the receptive field center. We made extracellular and whole-cell recordings of guinea pig Y-type cells in vitro and quantified the gain change between contrasts using a linear-nonlinear analysis. For spikes, tripling contrast reduced gain by approximately 40%. With spikes blocked, ganglion cells showed similar levels of gain control in membrane currents and voltages and under conditions of low and high calcium buffering: tripling contrast reduced gain by approximately 20-25%. Gain control persisted under voltage-clamp conditions that minimize inhibitory conductances and pharmacological conditions that block inhibitory neurotransmitter receptors. Gain control depended on adequate stimulation, not of ganglion cells but of presynaptic bipolar cells. Furthermore, horizontal cell measurements showed a lack of gain control in photoreceptor synaptic release. Thus, the mechanism for gain control over the ganglion cell receptive field center, as measured in the subthreshold response, originates in the presynaptic bipolar cells and does not require amacrine cell signaling.
Figures

Similar articles
-
Functional circuitry for peripheral suppression in Mammalian Y-type retinal ganglion cells.J Neurophysiol. 2007 Jun;97(6):4327-40. doi: 10.1152/jn.01091.2006. Epub 2007 Apr 25. J Neurophysiol. 2007. PMID: 17460102
-
Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell.J Physiol. 2008 Nov 15;586(22):5487-502. doi: 10.1113/jphysiol.2008.156224. Epub 2008 Oct 2. J Physiol. 2008. PMID: 18832424 Free PMC article.
-
The Synaptic and Morphological Basis of Orientation Selectivity in a Polyaxonal Amacrine Cell of the Rabbit Retina.J Neurosci. 2015 Sep 30;35(39):13336-50. doi: 10.1523/JNEUROSCI.1712-15.2015. J Neurosci. 2015. PMID: 26424882 Free PMC article.
-
How voltage-gated ion channels alter the functional properties of ganglion and amacrine cell dendrites.Arch Ital Biol. 2002 Oct;140(4):347-59. Arch Ital Biol. 2002. PMID: 12228988 Review.
-
Functional circuitry of visual adaptation in the retina.J Physiol. 2008 Sep 15;586(18):4377-84. doi: 10.1113/jphysiol.2008.156638. Epub 2008 Jul 10. J Physiol. 2008. PMID: 18617564 Free PMC article. Review.
Cited by
-
Spatial segregation of adaptation and predictive sensitization in retinal ganglion cells.Neuron. 2013 Aug 7;79(3):541-54. doi: 10.1016/j.neuron.2013.06.011. Neuron. 2013. PMID: 23932000 Free PMC article.
-
Effects of fixational eye movements on retinal ganglion cell responses: a modelling study.Front Comput Neurosci. 2007 Nov 2;1:2. doi: 10.3389/neuro.10.002.2007. eCollection 2007. Front Comput Neurosci. 2007. PMID: 18946524 Free PMC article.
-
Selective synaptic connections in the retinal pathway for night vision.J Comp Neurol. 2019 Jan 1;527(1):117-132. doi: 10.1002/cne.24313. Epub 2017 Sep 15. J Comp Neurol. 2019. PMID: 28856684 Free PMC article.
-
Receptoral Mechanisms for Fast Cholinergic Transmission in Direction-Selective Retinal Circuitry.Front Cell Neurosci. 2020 Nov 26;14:604163. doi: 10.3389/fncel.2020.604163. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33324168 Free PMC article.
-
Neurons in primary visual cortex represent distribution of luminance.Physiol Rep. 2016 Sep;4(18):e12966. doi: 10.14814/phy2.12966. Physiol Rep. 2016. PMID: 27655797 Free PMC article.
References
-
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. - PubMed
-
- Baccus SA, Meister M. Retina versus cortex; contrast adaptation in parallel visual pathways. Neuron. 2004;42:5–7. - PubMed
-
- Benardete EA, Kaplan E. The dynamics of primate M retinal ganglion cells. Vis Neurosci. 1999;16:355–368. - PubMed
-
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Vis Neurosci. 1992;8:483–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
