Transmission of spike trains at the retinogeniculate synapse
- PMID: 17344406
- PMCID: PMC6672514
- DOI: 10.1523/JNEUROSCI.5077-06.2007
Transmission of spike trains at the retinogeniculate synapse
Abstract
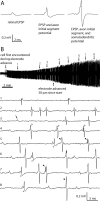
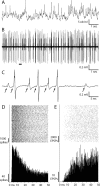
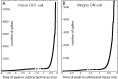
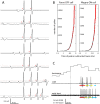
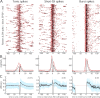
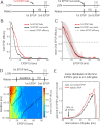
Retinal spikes impinging on relay neurons in the lateral geniculate nucleus (LGN) generate synaptic potentials, which sometimes produce spikes sent to visual cortex. We examined how signal transmission is regulated in the macaque LGN by recording the retinal input to a single LGN neuron while stimulating the receptive field center with a naturalistic luminance sequence. After extracting the EPSPs, which are often partially merged with spike waveforms, we found that >95% of spikes were associated with an EPSP from a single retinal ganglion cell. Each spike within a "burst" train was generated by an EPSP, indicating that LGN bursts are inherited from retinal bursts. LGN neurons rarely fired unless at least two EPSPs summated within 40 ms. This facilitation in EPSP efficacy was followed by depression. If a spike was generated by the first EPSP in a pair, it did not alter the efficacy of the second EPSP. Hence, the timing of EPSPs arising from the primary retinal driver governs synaptic efficacy and provides the basis for successful retinogeniculate transmission.
Figures

References
-
- Alexander GM, Carden WB, Mu J, Kurukulasuriya NC, McCool BA, Nordskog BK, Friedman DP, Daunais JB, Grant KA, Godwin DW. The native T-type calcium current in relay neurons of the primate thalamus. Neuroscience. 2006;141:453–461. - PubMed
-
- Bishop PO, Burke W, Davis R. Synapse discharge by single fibre in mammalian visual system. Nature. 1958;182:728–730. - PubMed
-
- Blasco B, Avendano C, Cavada C. A stereological analysis of the lateral geniculate nucleus in adult Macaca nemestrina monkeys. Vis Neurosci. 1999;16:933–941. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
