Beta1 integrins control the formation of cell chains in the adult rostral migratory stream
- PMID: 17344408
- PMCID: PMC6672488
- DOI: 10.1523/JNEUROSCI.2991-06.2007
Beta1 integrins control the formation of cell chains in the adult rostral migratory stream
Abstract
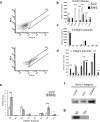
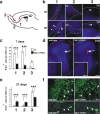
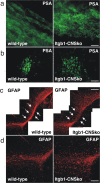
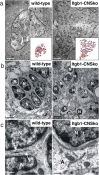
The subventricular zone (SVZ) of the lateral ventricle is the major site of neurogenesis in the adult brain. Neuroblasts that are born in the SVZ migrate as chains along the rostral migratory stream (RMS) to the olfactory bulb. Little is known about the mechanisms that control interactions between neuroblasts during their migration. Here we show that migrating neuroblasts express beta1 integrins and that the integrin ligand laminin is localized to cell chains. Using genetically modified mice and time-lapse video recordings of SVZ explants, we demonstrate that beta1 integrins and laminin promote the formation of cell chains. Laminin also induces the aggregation of purified neuroblasts. We conclude that the formation of cell chains in the RMS is controlled in part by beta1 integrins via binding to laminin. In addition, we provide evidence that beta1 class integrins are required for the maintenance of the glial tubes and that defects in the glial tubes lead to the ectopic migration of neuroblasts into the surrounding tissue.
Figures



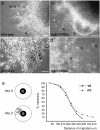
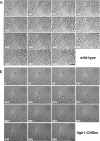
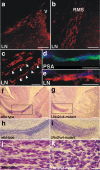

References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Andressen C, Adrian S, Fassler R, Arnhold S, Addicks K. The contribution of beta1 integrins to neuronal migration and differentiation depends on extracellular matrix molecules. Eur J Cell Biol. 2005;84:973–982. - PubMed
-
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
