Single chain Fab (scFab) fragment
- PMID: 17346344
- PMCID: PMC1829395
- DOI: 10.1186/1472-6750-7-14
Single chain Fab (scFab) fragment
Abstract
Background: The connection of the variable part of the heavy chain (VH) and and the variable part of the light chain (VL) by a peptide linker to form a consecutive polypeptide chain (single chain antibody, scFv) was a breakthrough for the functional production of antibody fragments in Escherichia coli. Being double the size of fragment variable (Fv) fragments and requiring assembly of two independent polypeptide chains, functional Fab fragments are usually produced with significantly lower yields in E. coli. An antibody design combining stability and assay compatibility of the fragment antigen binding (Fab) with high level bacterial expression of single chain Fv fragments would be desirable. The desired antibody fragment should be both suitable for expression as soluble antibody in E. coli and antibody phage display.
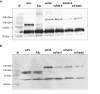
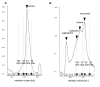
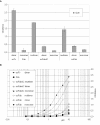
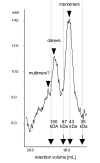
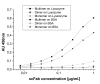
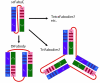
Results: Here, we demonstrate that the introduction of a polypeptide linker between the fragment difficult (Fd) and the light chain (LC), resulting in the formation of a single chain Fab fragment (scFab), can lead to improved production of functional molecules. We tested the impact of various linker designs and modifications of the constant regions on both phage display efficiency and the yield of soluble antibody fragments. A scFab variant without cysteins (scFabDeltaC) connecting the constant part 1 of the heavy chain (CH1) and the constant part of the light chain (CL) were best suited for phage display and production of soluble antibody fragments. Beside the expression system E. coli, the new antibody format was also expressed in Pichia pastoris. Monovalent and divalent fragments (DiFabodies) as well as multimers were characterised.
Conclusion: A new antibody design offers the generation of bivalent Fab derivates for antibody phage display and production of soluble antibody fragments. This antibody format is of particular value for high throughput proteome binder generation projects, due to the avidity effect and the possible use of common standard sera for detection.
Figures

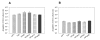
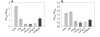


References
-
- Huston JS, Levinson D, Mudgett HM, Tai MS, Novotny J, Margolies MN, Ridge RJ, Bruccoloreri RE, Haber E, Crea R, Oppermann H. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digosin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. - DOI - PMC - PubMed
-
- Marks JD, Hoogenboom HR, Griffiths AD, Winter G. Molecular evolution of proteins on filamentous phage. J Biol Chem. 1992;267:16007–16010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

