The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse
- PMID: 17346997
- PMCID: PMC2763191
- DOI: 10.1016/j.immuni.2007.01.013
The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse
Abstract
T cell activation is predicated on the interaction between the T cell receptor and peptide-major histocompatibility (pMHC) ligands. The factors that determine the stimulatory potency of a pMHC molecule remain unclear. We describe results showing that a peptide exhibiting many hallmarks of a weak agonist stimulates T cells to proliferate more than the wild-type agonist ligand. An in silico approach suggested that the inability to form the central supramolecular activation cluster (cSMAC) could underlie the increased proliferation. This conclusion was supported by experiments that showed that enhancing cSMAC formation reduced stimulatory capacity of the weak peptide. Our studies highlight the fact that a complex interplay of factors determines the quality of a T cell antigen.
Figures



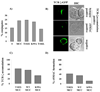
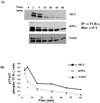
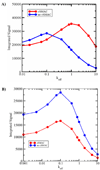
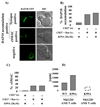
References
-
- Bachmann MF, Oxenius A, Speiser DE, Mariathasan S, Hengartner H, Zinkernagel RM, Ohashi PS. Peptide-induced T cell receptor down-regulation on naive T cells predicts agonist/partial agonist properties and strictly correlates with T cell activation. Eur J Immunol. 1997;27:2195–2203. - PubMed
-
- Berg LJ, Pullen AM, Fazekas de St Groth B, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. - PubMed
-
- Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

