Auto-inhibition of rat parallel fibre-Purkinje cell synapses by activity-dependent adenosine release
- PMID: 17347275
- PMCID: PMC2075183
- DOI: 10.1113/jphysiol.2006.126417
Auto-inhibition of rat parallel fibre-Purkinje cell synapses by activity-dependent adenosine release
Abstract
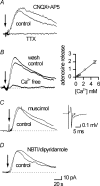
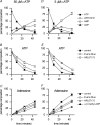
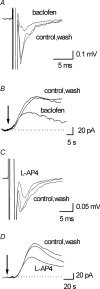
Adenosine is an important signalling molecule involved in a large number of physiological functions. In the brain these processes are as diverse as sleep, memory, locomotion and neuroprotection during episodes of ischaemia and hypoxia. Although the actions of adenosine, through cell surface G-protein-coupled receptors, are well characterized, in many cases the sources of adenosine and mechanisms of release have not been defined. Here we demonstrate the activity-dependent release of adenosine in the cerebellum using a combination of electrophysiology and biosensors. Short trains of electrical stimuli delivered to the molecular layer in vitro, release adenosine via a process that is both TTX and Ca(2+) sensitive. As ATP release cannot be detected, adenosine must either be released directly or rapidly produced by highly localized and efficient extracellular ATP breakdown. Since adenosine release can be modulated by receptors that act on parallel fibre-Purkinje cell synapses, we suggest that the parallel fibres release adenosine. This activity-dependent adenosine release exerts feedback inhibition of parallel fibre-Purkinje cell transmission. Spike-mediated adenosine release from parallel fibres will thus powerfully regulate cerebellar circuit output.
Figures




Similar articles
-
Adenosine signalling at immature parallel fibre-Purkinje cell synapses in rat cerebellum.J Physiol. 2009 Sep 15;587(Pt 18):4497-508. doi: 10.1113/jphysiol.2009.176420. Epub 2009 Aug 3. J Physiol. 2009. PMID: 19651764 Free PMC article.
-
Control of basal extracellular adenosine concentration in rat cerebellum.J Physiol. 2007 Jul 1;582(Pt 1):137-51. doi: 10.1113/jphysiol.2007.132050. Epub 2007 Apr 19. J Physiol. 2007. PMID: 17446223 Free PMC article.
-
Dynamics of Ca(2+) and Na(+) in the dendrites of mouse cerebellar Purkinje cells evoked by parallel fibre stimulation.Eur J Neurosci. 2003 Nov;18(10):2677-89. doi: 10.1111/j.1460-9568.2003.02977.x. Eur J Neurosci. 2003. PMID: 14656316
-
Ectopic release of glutamate contributes to spillover at parallel fibre synapses in the cerebellum.J Physiol. 2014 Apr 1;592(7):1493-503. doi: 10.1113/jphysiol.2013.267039. Epub 2014 Jan 13. J Physiol. 2014. PMID: 24421351 Free PMC article.
-
Modulation of synaptic activity in Purkinje neurons by ATP.Cerebellum. 2006;5(1):49-54. doi: 10.1080/14734220500497456. Cerebellum. 2006. PMID: 16527764 Review.
Cited by
-
Purinergic signaling in the retina: From development to disease.Brain Res Bull. 2019 Sep;151:92-108. doi: 10.1016/j.brainresbull.2018.10.016. Epub 2018 Nov 17. Brain Res Bull. 2019. PMID: 30458250 Free PMC article. Review.
-
Adenosine A₂a receptors and O₂ sensing in development.Am J Physiol Regul Integr Comp Physiol. 2011 Sep;301(3):R601-22. doi: 10.1152/ajpregu.00664.2010. Epub 2011 Jun 15. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21677265 Free PMC article. Review.
-
Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation.J Cell Biol. 2009 Jul 13;186(1):85-97. doi: 10.1083/jcb.200901084. J Cell Biol. 2009. PMID: 19596849 Free PMC article.
-
Adenosine signalling at immature parallel fibre-Purkinje cell synapses in rat cerebellum.J Physiol. 2009 Sep 15;587(Pt 18):4497-508. doi: 10.1113/jphysiol.2009.176420. Epub 2009 Aug 3. J Physiol. 2009. PMID: 19651764 Free PMC article.
-
Neuromodulation at single presynaptic boutons of cerebellar parallel fibers is determined by bouton size and basal action potential-evoked Ca transient amplitude.J Neurosci. 2009 Dec 9;29(49):15586-94. doi: 10.1523/JNEUROSCI.3793-09.2009. J Neurosci. 2009. PMID: 20007482 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

