The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis
- PMID: 17347684
- PMCID: PMC1810574
- DOI: 10.1172/JCI30182
The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis
Abstract
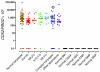
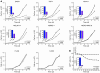
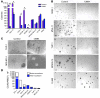
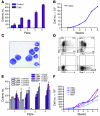
The homeobox transcription factor CDX2 plays an important role in embryonic development and regulates the proliferation and differentiation of intestinal epithelial cells in the adult. We have found that CDX2 is expressed in leukemic cells of 90% of patients with acute myeloid leukemia (AML) but not in hematopoietic stem and progenitor cells derived from normal individuals. Stable knockdown of CDX2 expression by RNA interference inhibited the proliferation of various human AML cell lines and strongly reduced their clonogenic potential in vitro. Primary murine hematopoietic progenitor cells transduced with Cdx2 acquired serial replating activity, were able to be continuously propagated in liquid culture, generated fully penetrant and transplantable AML in BM transplant recipients, and displayed dysregulated expression of Hox family members in vitro and in vivo. These results demonstrate that aberrant expression of the developmental regulatory gene CDX2 in the adult hematopoietic compartment is a frequent event in the pathogenesis of AML; suggest a role for CDX2 as part of a common effector pathway that promotes the proliferative capacity and self-renewal potential of myeloid progenitor cells; and support the hypothesis that CDX2 is responsible, in part, for the altered HOX gene expression that is observed in most cases of AML.
Figures


Comment in
-
HOX deregulation in acute myeloid leukemia.J Clin Invest. 2007 Apr;117(4):865-8. doi: 10.1172/JCI31861. J Clin Invest. 2007. PMID: 17404613 Free PMC article.
References
-
- Abramovich C., Humphries R.K. Hox regulation of normal and leukemic hematopoietic stem cells. Curr. Opin. Hematol. 2005;12:210–216. - PubMed
-
- Deschamps J., et al. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int. J. Dev. Biol. 1999;43:635–650. - PubMed
-
- van den Akker E., et al. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129:2181–2193. - PubMed
-
- Charite J., et al. Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development. 1998;125:4349–4358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

