Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts
- PMID: 17347686
- PMCID: PMC1810571
- DOI: 10.1172/JCI29713
Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts
Abstract
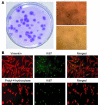
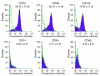
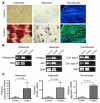
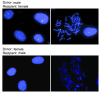
The origin and turnover of connective tissue cells in adult human organs, including the lung, are not well understood. Here, studies of cells derived from human lung allografts demonstrate the presence of a multipotent mesenchymal cell population, which is locally resident in the human adult lung and has extended life span in vivo. Examination of plastic-adherent cell populations in bronchoalveolar lavage samples obtained from 76 human lung transplant recipients revealed clonal proliferation of fibroblast-like cells in 62% (106 of 172) of samples. Immunophenotyping of these isolated cells demonstrated expression of vimentin and prolyl-4-hydroxylase, indicating a mesenchymal phenotype. Multiparametric flow cytometric analyses revealed expression of cell-surface proteins, CD73, CD90, and CD105, commonly found on mesenchymal stem cells (MSCs). Hematopoietic lineage markers CD14, CD34, and CD45 were absent. Multipotency of these cells was demonstrated by their capacity to differentiate into adipocytes, chondrocytes, and osteocytes. Cytogenetic analysis of cells from 7 sex-mismatched lung transplant recipients harvested up to 11 years after transplant revealed that 97.2% +/- 2.1% expressed the sex genotype of the donor. The presence of MSCs of donor sex identity in lung allografts even years after transplantation provides what we believe to be the first evidence for connective tissue cell progenitors that reside locally within a postnatal, nonhematopoietic organ.
Figures
References
-
- Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Gerson S.L. Mesenchymal stem cells: no longer second class marrow citizens. Nat. Med. 1999;5:262–264. - PubMed
-
- Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

