Human mass balance study of the novel anticancer agent ixabepilone using accelerator mass spectrometry
- PMID: 17347871
- PMCID: PMC1915607
- DOI: 10.1007/s10637-007-9041-z
Human mass balance study of the novel anticancer agent ixabepilone using accelerator mass spectrometry
Abstract
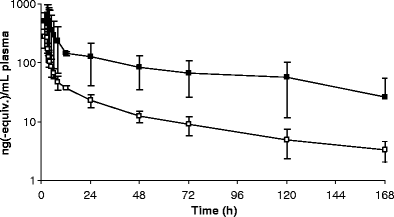
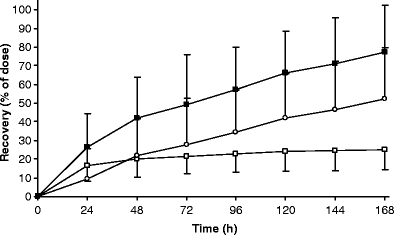
Ixabepilone (BMS-247550) is a semi-synthetic, microtubule stabilizing epothilone B analogue which is more potent than taxanes and has displayed activity in taxane-resistant patients. The human plasma pharmacokinetics of ixabepilone have been described. However, the excretory pathways and contribution of metabolism to ixabepilone elimination have not been determined. To investigate the elimination pathways of ixabepilone we initiated a mass balance study in cancer patients. Due to autoradiolysis, ixabepilone proved to be very unstable when labeled with conventional [14C]-levels (100 microCi in a typical human radio-tracer study). This necessitated the use of much lower levels of [14C]-labeling and an ultra-sensitive detection method, Accelerator Mass Spectrometry (AMS). Eight patients with advanced cancer (3 males, 5 females; median age 54.5 y; performance status 0-2) received an intravenous dose of 70 mg, 80 nCi of [14C]ixabepilone over 3 h. Plasma, urine and faeces were collected up to 7 days after administration and total radioactivity (TRA) was determined using AMS. Ixabepilone in plasma and urine was quantitated using a validated LC-MS/MS method. Mean recovery of ixabepilone-derived radioactivity was 77.3% of dose. Fecal excretion was 52.2% and urinary excretion was 25.1%. Only a minor part of TRA is accounted for by unchanged ixabepilone in both plasma and urine, which indicates that metabolism is a major elimination mechanism for this drug. Future studies should focus on structural elucidation of ixabepilone metabolites and characterization of their activities.
Figures



Similar articles
-
Biotransformation profiling of [(14)C]ixabepilone in human plasma, urine and feces samples using accelerator mass spectrometry (AMS).Drug Metab Pharmacokinet. 2009;24(6):511-22. doi: 10.2133/dmpk.24.511. Drug Metab Pharmacokinet. 2009. PMID: 20045986
-
Liquid chromatography and tandem mass spectrometry for the quantitative determination of ixabepilone (BMS-247550, Ixempra) in human plasma: method validation, overcoming curve splitting issues and eliminating chromatographic interferences from degradants.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Feb 15;878(5-6):525-37. doi: 10.1016/j.jchromb.2009.12.014. Epub 2009 Dec 16. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 20083444
-
Preclinical discovery of ixabepilone, a highly active antineoplastic agent.Cancer Chemother Pharmacol. 2008 Dec;63(1):157-66. doi: 10.1007/s00280-008-0724-8. Epub 2008 Mar 18. Cancer Chemother Pharmacol. 2008. PMID: 18347795 Review.
-
In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models.Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):6950-8. doi: 10.1158/1078-0432.CCR-05-0740. Clin Cancer Res. 2005. PMID: 16203787
-
Clinical development of ixabepilone and other epothilones in patients with advanced solid tumors.Oncologist. 2008 Dec;13(12):1207-23. doi: 10.1634/theoncologist.2008-0143. Epub 2008 Dec 16. Oncologist. 2008. PMID: 19088324 Review.
Cited by
-
Dose selection method for pharmacokinetic study in hemodialysis patients using a subpharmacological dose: oseltamivir as a model drug.BMC Nephrol. 2014 Mar 17;15:46. doi: 10.1186/1471-2369-15-46. BMC Nephrol. 2014. PMID: 24636040 Free PMC article. Clinical Trial.
-
Novel microtubule-targeting agents - the epothilones.Biologics. 2008 Dec;2(4):789-811. doi: 10.2147/btt.s3487. Biologics. 2008. PMID: 19707459 Free PMC article.
-
Pharmacokinetics, absorption, and excretion of radiolabeled revexepride: a Phase I clinical trial using a microtracer and accelerator mass spectrometry-based approach.Drug Des Devel Ther. 2016 Sep 27;10:3125-3132. doi: 10.2147/DDDT.S107843. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27729771 Free PMC article. Clinical Trial.
-
Human mass balance study of TAS-102 using (14)C analyzed by accelerator mass spectrometry.Cancer Chemother Pharmacol. 2016 Mar;77(3):515-26. doi: 10.1007/s00280-016-2965-2. Epub 2016 Jan 19. Cancer Chemother Pharmacol. 2016. PMID: 26787503 Free PMC article. Clinical Trial.
-
A Phase I Clinical, Pharmacokinetic, and Pharmacodynamic Study of Weekly or Every Three Week Ixabepilone and Daily Sunitinib in Patients with Advanced Solid Tumors.Clin Cancer Res. 2016 Jul 1;22(13):3209-17. doi: 10.1158/1078-0432.CCR-15-2184. Epub 2016 Feb 10. Clin Cancer Res. 2016. PMID: 26864210 Free PMC article. Clinical Trial.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1146/annurev.med.48.1.353', 'is_inner': False, 'url': 'https://doi.org/10.1146/annurev.med.48.1.353'}, {'type': 'PubMed', 'value': '9046968', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9046968/'}]}
- Rowinsky EK (1997) The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med 48:353–374 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '12415808', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12415808/'}]}
- Felip E (2002) New anti-tubulin agents. Suppl Tumori 1:S17–S18 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '7757983', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7757983/'}]}
- Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM (1995) Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 55:2325–2333 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1126/science.1099190', 'is_inner': False, 'url': 'https://doi.org/10.1126/science.1099190'}, {'type': 'PubMed', 'value': '15297674', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15297674/'}]}
- Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH (2004) The binding mode of epothilone A on alpha,beta-tubulin by electron crystallography. Science 305:866–869 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.272.27.17118', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.272.27.17118'}, {'type': 'PubMed', 'value': '9202030', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9202030/'}]}
- Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, Poruchynsky MS (1997) Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem 272:17118–17125 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

