What motivates British parents to consent for research? A questionnaire study
- PMID: 17349034
- PMCID: PMC1828728
- DOI: 10.1186/1471-2431-7-12
What motivates British parents to consent for research? A questionnaire study
Abstract
Background: Informed consent is the backbone of a clinical trial. In children this is given by their parents. There have been many studies in the neonatal population but little is known about the views of the parents of infants and young children from within the United Kingdom. The objectives of this study were to assess what motivates parents to consent to a randomised clinical trial (RCT), their feelings on consent and participation and the factors that would influence their decision to take part in a future study.
Methods: The setting was a multi-centre randomised but non-blinded equivalence trial of oral versus intravenous (IV) treatment for community acquired pneumonia in previously well children aged 6 months to 16 years in the UK (PIVOT Study). Parents were sent a postal questionnaire at the end of the study which included open and closed-ended questions. Fishers Exact Test was used to analyse associations in non parametric categorical data.
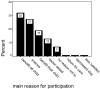
Results: 243 children were recruited into the PIVOT study. Of a possible 235, 136 questionnaires were returned (response rate 59%). Of those questionnaires returned; 98% of parents remembered consenting, 95% felt they were given enough time to make their decision and 96% felt they received enough information. Major reasons for participation were benefit to other children in the future 31%, contribution to science 27%, benefit to their own child 18%. Most parents (85%) did not feel obliged to participate. 62% felt there was an advantage to taking part and 18% felt there was a disadvantage. 91% of parents said they would take part in a similar study in the future, stating influences on their decision being benefit to their own child (91%) and benefit to all children (89%).
Conclusion: The major motivation in parents consenting for their previously well child to participate in an RCT of therapy for an acute medical illness was to increase medical knowledge in the future. Most saw an advantage in taking part in the trial and did not feel obliged to participate.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources