Protein kinase C delta is required for survival of cells expressing activated p21RAS
- PMID: 17350960
- PMCID: PMC3527128
- DOI: 10.1074/jbc.M610225200
Protein kinase C delta is required for survival of cells expressing activated p21RAS
Abstract
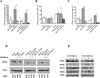
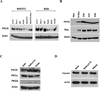
Inhibition of protein kinase C (PKC) activity in transformed cells and tumor cells containing activated p21(RAS) results in apoptosis. To investigate the pro-apoptotic pathway induced by the p21(RAS) oncoprotein, we first identified the specific PKC isozyme necessary to prevent apoptosis in the presence of activated p21(RAS). Dominant-negative mutants of PKC, short interfering RNA vectors, and PKC isozyme-specific chemical inhibitors directed against the PKCdelta isozyme demonstrated that PKCdelta plays a critical role in p21(RAS)-mediated apoptosis. An activating p21(RAS) mutation, or activation of the phosphatidylinositol 3-kinase (PI3K) Ras effector pathway, increased the levels of PKCdelta protein and activity in cells, whereas inhibition of p21(RAS) activity decreased the expression of the PKCdelta protein. Activation of the Akt survival pathway by oncogenic Ras required PKCdelta activity. Akt activity was dramatically decreased after PKCdelta suppression in cells containing activated p21(RAS). Conversely, constitutively activated Akt rescued cells from apoptosis induced by PKCdelta inhibition. Collectively, these findings demonstrate that p21(RAS), through its downstream effector PI3K, induces PKCdelta expression and that this increase in PKCdelta activity, acting through Akt, is required for cell survival. The p21(RAS) effector molecule responsible for the initiation of the apoptotic signal after suppression of PKCdelta activity was also determined to be PI3K. PI3K (p110(C)(AAX), where AA is aliphatic amino acid) was sufficient for induction of apoptosis after PKCdelta inhibition. Thus, the same p21(RAS) effector, PI3K, is responsible for delivering both a pro-apoptotic signal and a survival signal, the latter being mediated by PKCdelta and Akt. Selective suppression of PKCdelta activity and consequent induction of apoptosis is a potential strategy for targeting of tumor cells containing an activated p21(RAS).
Figures









Similar articles
-
PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1.Cell Signal. 2009 Apr;21(4):502-8. doi: 10.1016/j.cellsig.2008.12.002. Epub 2008 Dec 10. Cell Signal. 2009. PMID: 19146951 Free PMC article.
-
Divergence in the anti-apoptotic signalling pathways used by nerve growth factor and basic fibroblast growth factor (bFGF) in PC12 cells: rescue by bFGF involves protein kinase C delta.Biochem J. 2000 Nov 15;352 Pt 1(Pt 1):175-82. Biochem J. 2000. PMID: 11062070 Free PMC article.
-
Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B.Mol Cell Biol. 2003 Apr;23(7):2251-63. doi: 10.1128/MCB.23.7.2251-2263.2003. Mol Cell Biol. 2003. PMID: 12640111 Free PMC article.
-
Role of RAS signaling in ovarian cancer.F1000Res. 2022 Nov 4;11:1253. doi: 10.12688/f1000research.126337.1. eCollection 2022. F1000Res. 2022. PMID: 36451660 Free PMC article. Review.
-
Molecular pathways: targeting RAC-p21-activated serine-threonine kinase signaling in RAS-driven cancers.Clin Cancer Res. 2014 Sep 15;20(18):4740-6. doi: 10.1158/1078-0432.CCR-13-1727. Clin Cancer Res. 2014. PMID: 25225063 Free PMC article. Review.
Cited by
-
Protein kinase C-delta mediates neuronal apoptosis in the retinas of diabetic rats via the Akt signaling pathway.Diabetes. 2008 Aug;57(8):2181-90. doi: 10.2337/db07-1431. Epub 2008 Apr 28. Diabetes. 2008. PMID: 18443201 Free PMC article.
-
PI3K Acts in synergy with loss of PKC to elicit apoptosis via the UPR.J Cell Biochem. 2009 May 1;107(1):76-85. doi: 10.1002/jcb.22102. J Cell Biochem. 2009. PMID: 19241442 Free PMC article.
-
Protein kinase Cdelta supports survival of MDA-MB-231 breast cancer cells by suppressing the ERK1/2 pathway.J Biol Chem. 2009 Nov 27;284(48):33456-65. doi: 10.1074/jbc.M109.036186. Epub 2009 Oct 15. J Biol Chem. 2009. PMID: 19833733 Free PMC article.
-
Protein kinase Cδ inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors.Endocr Relat Cancer. 2011 Dec 1;18(6):759-71. doi: 10.1530/ERC-10-0224. Print 2011 Dec. Endocr Relat Cancer. 2011. PMID: 21990324 Free PMC article.
-
Pleiotropic Roles of Calmodulin in the Regulation of KRas and Rac1 GTPases: Functional Diversity in Health and Disease.Int J Mol Sci. 2020 May 23;21(10):3680. doi: 10.3390/ijms21103680. Int J Mol Sci. 2020. PMID: 32456244 Free PMC article. Review.
References
-
- Wiesmuller L, Wittinghofer F. Cell Signal. 1994;6(3):247–267. - PubMed
-
- Adjei AA. J Natl Cancer Inst. 2001;93(14):1062–1074. - PubMed
-
- Downward J. Nat Rev Cancer. 2003;3(1):11–22. - PubMed
-
- Thimmaiah KN, Easton J, Huang S, Veverka KA, Germain GS, Harwood FC, Houghton PJ. Cancer Res. 2003;63(2):364–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

