Lipid chain-length dependence for incorporation of alamethicin in membranes: electron paramagnetic resonance studies on TOAC-spin labeled analogs
- PMID: 17351010
- PMCID: PMC1868974
- DOI: 10.1529/biophysj.107.104026
Lipid chain-length dependence for incorporation of alamethicin in membranes: electron paramagnetic resonance studies on TOAC-spin labeled analogs
Abstract
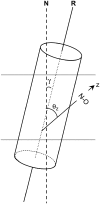
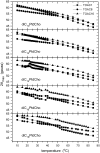
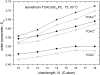
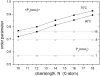
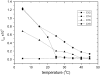
Alamethicin is a 19-residue hydrophobic peptide, which is extended by a C-terminal phenylalaninol but lacks residues that might anchor the ends of the peptide at the lipid-water interface. Voltage-dependent ion channels formed by alamethicin depend strongly in their characteristics on chain length of the host lipid membranes. EPR spectroscopy is used to investigate the dependence on lipid chain length of the incorporation of spin-labeled alamethicin in phosphatidylcholine bilayer membranes. The spin-label amino acid TOAC is substituted at residue positions n = 1, 8, or 16 in the sequence of alamethicin F50/5 [TOAC(n), Glu(OMe)(7,18,19)]. Polarity-dependent isotropic hyperfine couplings of the three TOAC derivatives indicate that alamethicin assumes approximately the same location, relative to the membrane midplane, in fluid diC(N)PtdCho bilayers with chain lengths ranging from N = 10-18. Residue TOAC(8) is situated closest to the bilayer midplane, whereas TOAC(16) is located farther from the midplane in the hydrophobic core of the opposing lipid leaflet, and TOAC(1) remains in the lipid polar headgroup region. Orientational order parameters indicate that the tilt of alamethicin relative to the membrane normal is relatively small, even at high temperatures in the fluid phase, and increases rather slowly with decreasing chain length (from 13 degrees to 23 degrees for N = 18 and 10, respectively, at 75 degrees C). This is insufficient for alamethicin to achieve hydrophobic matching. Alamethicin differs in its mode of incorporation from other helical peptides for which transmembrane orientation has been determined as a function of lipid chain length.
Figures
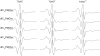





Similar articles
-
Intramembrane water associated with TOAC spin-labeled alamethicin: electron spin-echo envelope modulation by D2O.Biophys J. 2009 Feb;96(3):997-1007. doi: 10.1016/j.bpj.2008.10.024. Biophys J. 2009. PMID: 19186137 Free PMC article.
-
TOAC spin labels in the backbone of alamethicin: EPR studies in lipid membranes.Biophys J. 2007 Jan 15;92(2):473-81. doi: 10.1529/biophysj.106.092775. Epub 2006 Oct 20. Biophys J. 2007. PMID: 17056731 Free PMC article.
-
Orientation and peptide-lipid interactions of alamethicin incorporated in phospholipid membranes: polarized infrared and spin-label EPR spectroscopy.Biochemistry. 2009 Feb 3;48(4):729-37. doi: 10.1021/bi801279n. Biochemistry. 2009. PMID: 19133787
-
Voltage-dependent channels in planar lipid bilayer membranes.Physiol Rev. 1981 Jan;61(1):77-150. doi: 10.1152/physrev.1981.61.1.77. Physiol Rev. 1981. PMID: 6258181 Review. No abstract available.
-
Biological and model membranes studied by nuclear magnetic resonance of spin one half nuclei.Q Rev Biophys. 1977 Feb;10(1):67-96. doi: 10.1017/s0033583500000147. Q Rev Biophys. 1977. PMID: 327502 Review. No abstract available.
Cited by
-
Structure of self-aggregated alamethicin in ePC membranes detected by pulsed electron-electron double resonance and electron spin echo envelope modulation spectroscopies.Biophys J. 2009 Apr 22;96(8):3197-209. doi: 10.1016/j.bpj.2009.01.026. Biophys J. 2009. PMID: 19383464 Free PMC article.
-
Energetics of hydrophobic matching in lipid-protein interactions.Biophys J. 2008 May 15;94(10):3996-4013. doi: 10.1529/biophysj.107.121475. Epub 2008 Jan 30. Biophys J. 2008. PMID: 18234817 Free PMC article.
-
Lipid Fluid-Gel Phase Transition Induced Alamethicin Orientational Change Probed by Sum Frequency Generation Vibrational Spectroscopy.J Phys Chem C Nanomater Interfaces. 2013 Aug 20;117(33):17039-17049. doi: 10.1021/jp4047215. J Phys Chem C Nanomater Interfaces. 2013. PMID: 24124624 Free PMC article.
-
Domain Size Regulation in Phospholipid Model Membranes Using Oil Molecules and Hybrid Lipids.J Phys Chem B. 2022 Aug 11;126(31):5842-5854. doi: 10.1021/acs.jpcb.2c02862. Epub 2022 Jul 27. J Phys Chem B. 2022. PMID: 35895895 Free PMC article.
-
Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes.Biophys J. 2007 Dec 1;93(11):3884-99. doi: 10.1529/biophysj.107.107938. Epub 2007 Aug 17. Biophys J. 2007. PMID: 17704167 Free PMC article.
References
-
- Fox, R. O. Jr., and F. M. Richards. 1982. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5 Å resolution. Nature. 300:325–330. - PubMed
-
- Nagaraj, R., and P. Balaram. 1981. Alamethicin, a transmembrane channel. Acc. Chem. Res. 14:356–362.
-
- Barranger-Mathys, M., and D. S. Cafiso. 1996. Membrane structure of voltage-gated channel forming peptides by site-directed spin-labeling. Biochemistry. 35:498–505. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

