Angiotensin-(1-7)-induced plasticity changes in the lateral amygdala are mediated by COX-2 and NO
- PMID: 17351141
- PMCID: PMC1838559
- DOI: 10.1101/lm.425907
Angiotensin-(1-7)-induced plasticity changes in the lateral amygdala are mediated by COX-2 and NO
Abstract
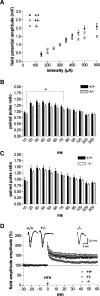
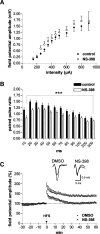
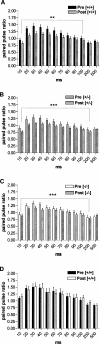
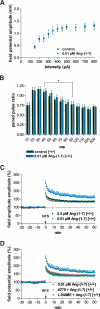
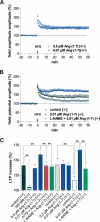
It is known from studies outside the brain that upon binding to its receptor, angiotensin-(1-7) elicits the release of prostanoids and nitric oxide (NO). Cyclooxygenase (COX) is a key enzyme that converts arachidonic acid to prostaglandins. Since there are no data available so far on the role of COX-2 in the amygdala, in a first step we demonstrated that the selective COX-2 inhibitor NS-398 significantly reduced the probability of long-term potentiation (LTP) induction in the lateral nucleus of the amygdala. Similarly, in COX-2(-/-) mice, LTP induced by external capsule (EC) stimulation was impaired. Second, we evaluated the action of angiotensin-(1-7) in the amygdala. In wild-type mice, angiotensin-(1-7) increased LTP. This LTP-enhancing effect of Ang-(1-7) was not observed in COX-2(+/-) mice. However, in COX-2(-/-) mice, Ang-(1-7) caused an enhancement of LTP similar to that in wild-type mice. The NO synthetase inhibitor L-NAME blocked this angiotensin-(1-7)-induced increase in LTP in COX-2(-/-) mice. Low-frequency stimulation of external capsule fibers did not cause long-term depression (LTD) in drug-free and angiotensin-(1-7)-treated brain slices in wild-type mice. In contrast, in COX-2(-/-) mice, angiotensin-(1-7) caused stable LTD. Increasing NO concentration by the NO-donor SNAP also caused LTD in wild-type mice. Our study shows for the first time that LTP in the amygdala is dependent on COX-2 activity. Moreover, COX-2 is involved in the mediation of angiotensin-(1-7) effects on LTP. Finally, it is recognized that there is a molecular cross-talk between COX-2 and NO that may regulate synaptic plasticity.
Figures

References
-
- Albrecht D., Nitschke T., von Bohlen und Halbach O. Various effects of angiotensin II on amygdaloid neuronal activity in normotensive control and hypertensive transgenic [TGR(mREN-2)27] rats. FASEB J. 2000;14:925–931. - PubMed
-
- Arima S., Ito S. New insights into actions of the renin-angiotensin system in the kidney: Concentrating on the Ang II receptors and the newly described Ang-(1-7) and its receptor. Semin. Nephrol. 2001;21:535–543. - PubMed
-
- Armstrong D.L., Garcia E.A., Ma T., Quinones B., Wayner M.J. Angiotensin-II blockade of long-term potentiation at the perforant path-granule cell synapse in-vitro. Peptides. 1996;17:689–693. - PubMed
-
- Bidmon H.J., Oermann E., Schiene K., Schmitt M., Kato K., Asayama K., Witte O.W., Zilles K. Unilateral upregulation of cyclooxygenase-2 following cerebral, cortical photothrombosis in the rat: Suppression by MK-801 and co-distribution with enzymes involved in the oxidative stress cascade. J. Chem. Neuroanat. 2000;20:163–176. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
