Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty
- PMID: 17351635
- PMCID: PMC1858651
- DOI: 10.1038/nn1868
Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty
Abstract
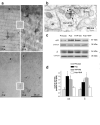
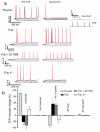
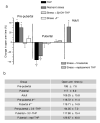
Puberty is characterized by mood swings and anxiety, which are often produced by stress. Here we show that THP (allopregnanolone), a steroid that is released as a result of stress, increases anxiety in pubertal female mice, in contrast to its anxiety-reducing effect in adults. Anxiety is regulated by GABAergic inhibition in limbic circuits. Although this inhibition is increased by THP administration before puberty and in adults, during puberty THP reduces the tonic inhibition of pyramidal cells in hippocampal region CA1, leading to increased excitability. This paradoxical effect of THP results from inhibition of alpha4betadelta GABAA receptors. These receptors are normally expressed at very low levels, but at puberty, their expression is increased in hippocampal area CA1, where they generate outward currents. THP also decreases the outward current at recombinant alpha4beta2delta receptors, and this effect depends on arginine 353 in the alpha4 subunit, a putative site for modulation by Cl-. Therefore, inhibition of alpha4beta2delta GABAA receptors by THP provides a mechanism for the generation of anxiety at puberty.
Figures




Comment in
-
GABA receptors make teens resistant to input.Nat Neurosci. 2007 Apr;10(4):397-9. doi: 10.1038/nn0407-397. Nat Neurosci. 2007. PMID: 17387324 No abstract available.
Similar articles
-
Puberty, steroids and GABA(A) receptor plasticity.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S91-S103. doi: 10.1016/j.psyneuen.2009.05.011. Psychoneuroendocrinology. 2009. PMID: 19523771 Free PMC article.
-
A stress steroid triggers anxiety via increased expression of α4βδ GABAA receptors in methamphetamine dependence.Neuroscience. 2013 Dec 19;254:452-75. doi: 10.1016/j.neuroscience.2013.08.033. Epub 2013 Aug 29. Neuroscience. 2013. PMID: 23994152 Free PMC article.
-
Neurosteroid effects at α4βδ GABAA receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse.Brain Res. 2015 Sep 24;1621:170-86. doi: 10.1016/j.brainres.2014.12.026. Epub 2014 Dec 24. Brain Res. 2015. PMID: 25542386 Free PMC article.
-
α4βδ GABAA receptors and tonic inhibitory current during adolescence: effects on mood and synaptic plasticity.Front Neural Circuits. 2013 Sep 3;7:135. doi: 10.3389/fncir.2013.00135. eCollection 2013. Front Neural Circuits. 2013. PMID: 24027497 Free PMC article. Review.
-
The influence of stress at puberty on mood and learning: role of the α4βδ GABAA receptor.Neuroscience. 2013 Sep 26;249:192-213. doi: 10.1016/j.neuroscience.2012.09.065. Epub 2012 Oct 16. Neuroscience. 2013. PMID: 23079628 Free PMC article. Review.
Cited by
-
Neurosteroid influences on sensitivity to ethanol.Front Endocrinol (Lausanne). 2012 Jan 31;3:10. doi: 10.3389/fendo.2012.00010. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22654852 Free PMC article.
-
α4-GABAA receptors of hippocampal pyramidal neurons are associated with resilience against activity-based anorexia for adolescent female mice but not for males.Mol Cell Neurosci. 2018 Apr 22;90:33-48. doi: 10.1016/j.mcn.2018.04.008. Online ahead of print. Mol Cell Neurosci. 2018. PMID: 29684457 Free PMC article.
-
The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells.J Physiol. 2008 Feb 15;586(4):989-1004. doi: 10.1113/jphysiol.2007.146746. Epub 2007 Dec 13. J Physiol. 2008. PMID: 18079158 Free PMC article.
-
A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder.Mol Psychiatry. 2008 Sep;13(9):829, 833-57. doi: 10.1038/mp.2008.65. Epub 2008 Jun 24. Mol Psychiatry. 2008. PMID: 18574483 Free PMC article. Review.
-
Excitatory synapses on dendritic shafts of the caudal basal amygdala exhibit elevated levels of GABAA receptor α4 subunits following the induction of activity-based anorexia.Synapse. 2014 Jan;68(1):1-15. doi: 10.1002/syn.21690. Epub 2013 Jul 30. Synapse. 2014. PMID: 23766101 Free PMC article.
References
-
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol. Bull. 1992;111:62–107. - PubMed
-
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J. Adolescent Health. 2002;30S:49–58. - PubMed
-
- Modesti PA, et al. Changes in blood pressure reactivity and 24-hour blood pressure profile occurring at puberty. Angiology. 2006;45:443–450. - PubMed
-
- Rudolph U, et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. - PubMed
-
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

