Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid
- PMID: 17351657
- PMCID: PMC2012973
- DOI: 10.1038/sj.bjp.0707198
Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid
Abstract
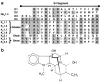
Background and purpose: Angiogenesis involves multiple signaling pathways that must be considered when developing agents to modulate pathological angiogenesis. Because both cyclooxygenase inhibitors and dithioles have demonstrated anti-angiogenic properties, we investigated the activities of a new class of anti-inflammatory drugs containing dithiolethione moieties (S-NSAIDs) and S-valproate.
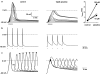
Experimental approach: Anti-angiogenic activities of S-NSAIDS, S-valproate, and the respective parent compounds were assessed using umbilical vein endothelial cells, muscle and tumor tissue explant angiogenesis assays, and developmental angiogenesis in Fli:EGFP transgenic zebrafish embryos.
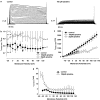
Key results: Dithiolethione derivatives of diclofenac, valproate, and sulindac inhibited endothelial cell proliferation and induced Ser(78) phosphorylation of hsp27, a known molecular target of anti-angiogenic signaling. The parent drugs lacked this activity, but dithiolethiones were active at comparable concentrations. Although dithiolethiones can potentially release hydrogen sulphide, NaSH did not reproduce some activities of the S-NSAIDs, indicating that the dithioles regulate angiogenesis through mechanisms other than release of H(2)S. In contrast to the parent drugs, S-NSAIDs, S-valproate, NaSH, and dithiolethiones were potent inhibitors of angiogenic responses in muscle and HT29 tumor explants assessed by 3-dimensional collagen matrix assays. Dithiolethiones and valproic acid were also potent inhibitors of developmental angiogenesis in zebrafish embryos, but the S-NSAIDs, remarkably, lacked this activity.
Conclusions and implication: S-NSAIDs and S-valproate have potent anti-angiogenic activities mediated by their dithiole moieties. The novel properties of S-NSAIDs and S-valproate to inhibit pathological versus developmental angiogenesis suggest that these agents may have a role in cancer treatment.
Figures






References
-
- Alvarez JL, Vassort G. Dual action of prajmaline on the Ca2+ currents in frog isolated cardiomyocytes. J Mol Cell Cardiol. 1991;23:627–638. - PubMed
-
- Bebarova M, Matejovic P, Pasek M, Simurdova M, Simurda J. Effect of ajmaline on transient outward current in rat ventricular myocytes. Gen Physiol Biophys. 2005a;24:27–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

