Correlation of apparent affinity values from H3-receptor binding assays with apparent affinity (pKapp) and intrinsic activity (alpha) from functional bioassays
- PMID: 17351664
- PMCID: PMC2012978
- DOI: 10.1038/sj.bjp.0707174
Correlation of apparent affinity values from H3-receptor binding assays with apparent affinity (pKapp) and intrinsic activity (alpha) from functional bioassays
Abstract
Background and purpose: Agonist apparent affinities (pK(I)') in histamine H(3)-receptor binding assays were higher than expected from apparent affinity values (pK(app)) estimated in bioassay. Here, we investigate whether the degree of pK(I)' overestimation is related to agonist intrinsic efficacy, by studying the effect of buffer composition on the pK(I)' of ligands with varying intrinsic activity.
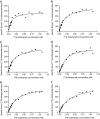
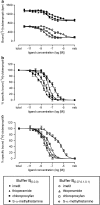
Experimental approach: In the guinea-pig ileum bioassay, intrinsic activity (alpha) was determined from the maximal inhibition of the contraction produced by increasing agonist concentration. pK(app) values were estimated using the method of Furchgott. The pK(L) of [(3)H]clobenpropit in guinea-pig cerebral cortex was estimated by saturation analysis in 20 mM HEPES-NaOH buffer (buffer B(0,0,0)), or buffer B(0,0,0) containing 70 mM CaCl(2), 100 mM NaCl and 100 mM KCl (buffer B(0.07,0.1,0.1)). PK(I) values were determined in competition studies in both buffers.
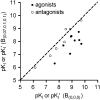
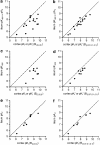
Key results: [(3)H]clobenpropit saturation isotherms had n (H) values of unity in both buffers. In buffer B(0.07,0.1,0.1), agonist pK(I)' values were closer to pK(app) values than in buffer B(0,0,0) but were associated with n (H) values <1. A two-site analysis of agonist data in buffer B(0.07, 0.1, 0.1) provided a better fit than a one-site fit and low affinity values (pK(IL)) were comparable to pK(app). Differences between the pK(I)' in buffer B(0,0,0) and pK(IL) values in buffer B(0.07,0.1,0.1) (DeltapK) were correlated with alpha.
Conclusions and implications: H(3)-receptor binding assays conducted in buffer B(0,0,0) and buffer B(0.07,0.1,0.1) can provide a measure of ligand affinity (pK(app)) and intrinsic efficacy. The assay predicts that some ligands previously classified as H(3)-receptor antagonists may possess residual intrinsic efficacy.
Figures




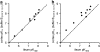


References
-
- Arias HR. Temperature and ionic strength dependence of quinacrine binding and quinacrine displacement elicited by high concentrations of agonists on the nicotinic acetylcholine receptor. Arch Biochem Biophys. 1996;333:1–11. - PubMed
-
- Arrang JM, Garbarg M, Lancelot J-C, Lecomte J-M, Pollard H, Robba M, et al. Highly potent and selective ligand for histamine H3-receptors. Nature. 1987;327:117–123. - PubMed
-
- Arrang J-M, Roy J, Morgat J-L, Schunack W, Schwartz J-C. Histamine H3 receptor binding sites in rat brain membranes: modulations by guanine nucleotides and divalent cations. Eur J Pharmacol. 1990;188:219–227. - PubMed
-
- Barnes JC, Brown JD, Clarke NP, Clapham J, Evans DJ, O'Shaughnessy CTO. Pharmacological activity of VUF9153, an isothiourea histamine H3 receptor antagonist. Eur J Pharmacol. 1993;250:147–152. - PubMed
-
- Black J. The Nobel Prizes 1988. Almqvist & Wiksell International: Stockholm Sweden; 1988. Drugs from emasculated hormones: the principles of syntopic antagonism.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

