Effects of hypothalamic paraventricular nuclei on apoptosis and proliferation of gastric mucosal cells induced by ischemia/reperfusion in rats
- PMID: 17352016
- PMCID: PMC4065922
- DOI: 10.3748/wjg.v13.i6.874
Effects of hypothalamic paraventricular nuclei on apoptosis and proliferation of gastric mucosal cells induced by ischemia/reperfusion in rats
Abstract
Aim: To investigate the effects of electrical stimulation of hypothalamic paraventricular nuclei (PVN) on gastric mucosal cellular apoptosis and proliferation induced by gastric ischemia/reperfusion (I/R) injury.
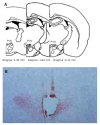
Methods: For different experimental purposes, stimulating electrode plantation or electrolytic destruction of the PVN was applied, then the animals' GI/R injury model was established by clamping the celiac artery for 30 min and allowing reperfusing the artery for 30 min, 1 h, 3 h or 6 h respectively. Then histological, immunohistochemistry methods were used to assess the gastric mucosal damage index, the gastric mucosal cellular apoptosis and proliferation at different times.
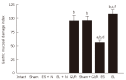
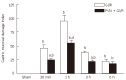
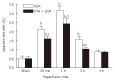
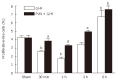
Results: The electrical stimulation of PVN significantly attenuated the GI/R injury at 30 min, 1 h and 3 h after reperfusion. The electrical stimulation of PVN decreased gastric mucosal apoptosis and increased gastric mucosal proliferation. The electrolytic destruction of the PVN could eliminate the protective effects of electrical stimulation of PVN on GI/R injury. These results indicated that the PVN participated in the regulation of GI/R injury as a specific area in the brain, exerting protective effects against the GI/R injury, and the protection was associated with the inhibition of cellular apoptosis and the promotion of gastric mucosal proliferation.
Conclusion: Stimulating PVN significantly inhibits the gastric mucosal cellular apoptosis and promots gastric mucosal cellular proliferation. This may explain the protective mechanisms of electrical stimulation of PVN against GI/R injury.
Figures


References
-
- De La Lastra CA, Cabeza J, Motilva V, Martin MJ. Melatonin protects against gastric ischemia-reperfusion injury in rats. J Pineal Res. 1997;23:47–52. - PubMed
-
- Kishimoto Y, Wada K, Nakamoto K, Ashida K, Kamisaki Y, Kawasaki H, Itoh T. Quantitative analysis of cyclooxygenase-2 gene expression on acute gastric injury induced by ischemia-reperfusion in rats. Life Sci. 1997;60:PL127–PL133. - PubMed
-
- Kitano M, Wada K, Kamisaki Y, Nakamoto K, Kishimoto Y, Kawasaki H, Itoh T. Effects of cimetidine on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Pharmacology. 1997;55:154–164. - PubMed
-
- Wada K, Kamisaki Y, Kitano M, Nakamoto K, Itoh T. Protective effect of cystathionine on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Eur J Pharmacol. 1995;294:377–382. - PubMed
-
- Andrews FJ, Malcontenti-Wilson C, O'Brien PE. Polymorphonuclear leukocyte infiltration into gastric mucosa after ischemia-reperfusion. Am J Physiol. 1994;266:G48–G54. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

