Conversion of the BASE prion strain into the BSE strain: the origin of BSE?
- PMID: 17352534
- PMCID: PMC1817656
- DOI: 10.1371/journal.ppat.0030031
Conversion of the BASE prion strain into the BSE strain: the origin of BSE?
Abstract
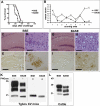
Atypical neuropathological and molecular phenotypes of bovine spongiform encephalopathy (BSE) have recently been identified in different countries. One of these phenotypes, named bovine "amyloidotic" spongiform encephalopathy (BASE), differs from classical BSE for the occurrence of a distinct type of the disease-associated prion protein (PrP), termed PrP(Sc), and the presence of PrP amyloid plaques. Here, we show that the agents responsible for BSE and BASE possess different biological properties upon transmission to transgenic mice expressing bovine PrP and inbred lines of nontransgenic mice. Strikingly, serial passages of the BASE strain to nontransgenic mice induced a neuropathological and molecular disease phenotype indistinguishable from that of BSE-infected mice. The existence of more than one agent associated with prion disease in cattle and the ability of the BASE strain to convert into the BSE strain may have important implications with respect to the origin of BSE and spongiform encephalopathies in other species, including humans.
Conflict of interest statement
Figures



References
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. - PubMed
-
- Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, et al. Cell-free formation of protease-resistant prion protein. Nature. 1994;370:419–420. - PubMed
-
- Castilla J, Saa P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. - PubMed
-
- Dickinson AG, Meikle VM. Host-genotype and agent effects in scrapie incubation: Change in allelic interaction with different strains of agent. Mol Gen Genet. 1971;112:73–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

