Dosage compensation is less effective in birds than in mammals
- PMID: 17352797
- PMCID: PMC2373894
- DOI: 10.1186/jbiol53
Dosage compensation is less effective in birds than in mammals
Abstract
Background: In animals with heteromorphic sex chromosomes, dosage compensation of sex-chromosome genes is thought to be critical for species survival. Diverse molecular mechanisms have evolved to effectively balance the expressed dose of X-linked genes between XX and XY animals, and to balance expression of X and autosomal genes. Dosage compensation is not understood in birds, in which females (ZW) and males (ZZ) differ in the number of Z chromosomes.
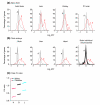
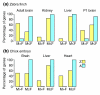
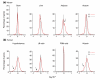
Results: Using microarray analysis, we compared the male:female ratio of expression of sets of Z-linked and autosomal genes in two bird species, zebra finch and chicken, and in two mammalian species, mouse and human. Male:female ratios of expression were significantly higher for Z genes than for autosomal genes in several finch and chicken tissues. In contrast, in mouse and human the male:female ratio of expression of X-linked genes is quite similar to that of autosomal genes, indicating effective dosage compensation even in humans, in which a significant percentage of genes escape X-inactivation.
Conclusion: Birds represent an unprecedented case in which genes on one sex chromosome are expressed on average at constitutively higher levels in one sex compared with the other. Sex-chromosome dosage compensation is surprisingly ineffective in birds, suggesting that some genomes can do without effective sex-specific sex-chromosome dosage compensation mechanisms.
Figures
Comment in
-
Does gene dosage really matter?J Biol. 2007;6(1):1. doi: 10.1186/jbiol55. J Biol. 2007. PMID: 17381829 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

