Plasmodium falciparum Na+/H+ exchanger activity and quinine resistance
- PMID: 17353059
- PMCID: PMC2747254
- DOI: 10.1016/j.molbiopara.2007.01.018
Plasmodium falciparum Na+/H+ exchanger activity and quinine resistance
Abstract
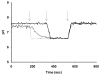
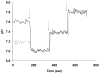
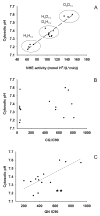
Mutations in the Plasmodium falciparum pfcrt gene cause resistance to the 4-amino quinoline chloroquine (CQ) and other antimalarial drugs. Mutations and/or overexpression of a P. falciparum multidrug resistance gene homologue (pfmdr1) may further modify or tailor the degree of quinoline drug resistance. Recently [Ferdig MT, Cooper RA, Mu JB, et al. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol Microbiol 2004;52:985-97] QTL analysis further implicated a region of P. falciparum chromosome 13 as a partner (with pfcrt) in conferring resistance to the first quinoline-based antimalarial drug, quinine (QN). Since QN resistance (QNR) and CQR are often (but not always) observed together in parasite strains, since elevated cytosolic pH is frequently (but not always) found in CQR parasites, and since the chr 13 segment linked to QNR prominently harbors a gene encoding what appears to be a P. falciparum Na(+)/H(+) exchanger (PfNHE), we have systematically measured cytosolic pH and PfNHE activity for an extended series of parasite strains used in the QTL analysis. Altered PfNHE activity does not correlate with CQR as previously proposed, but significantly elevated PfNHE activity is found for strains with high levels of QNR, regardless their CQR status. We propose that either an elevated pH(cyt) or a higher vacuolar pH-to-cytosolic pH gradient contributes to one common route to malarial QNR that is also characterized by recently defined chr 13-chr 9 pairwise interactions. Based on sequence analysis we propose a model whereby observed polymorphisms in PfNHE may lead to altered Na(+)/H(+) set point regulation in QNR parasites.
Figures
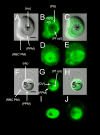



Comment in
-
P. falciparum Na(+)/H(+) exchanger (PfNHE) function and quinine resistance (QNR) [Reply to: Spillman et al. "Acid extrusion from the intraerythrocytic malaria parasite is not via a Na(+)/H(+) exchanger" Mol. Biochem. Parasitol. 2008 162 (1) 96-99].Mol Biochem Parasitol. 2009 Jul;166(1):1-2; author reply 3. doi: 10.1016/j.molbiopara.2009.01.008. Epub 2009 Apr 10. Mol Biochem Parasitol. 2009. PMID: 19428665 Free PMC article. No abstract available.
References
-
- Baird JK. Effectiveness of antimalarial drugs. N Engl J Med. 2005;352:1565–77. - PubMed
-
- Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LMB, Sidhu ABS, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mutations in the P-falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Molecular Cell. 2000;6:861–871. - PMC - PubMed
-
- Cooper RA, Ferdig MT, Su XZ, Ursos LM, Mu J, Nomura T, Fujioka H, Fidock DA, Roepe PD, Wellems TE. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum. Mol Pharmacol. 2002;61:35–42. - PubMed
-
- Sidhu AB, Valderramos SG, Fidock DA. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol Microbiol. 2005;57:913–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

