Bupropion SR for the treatment of smokeless tobacco use
- PMID: 17353101
- PMCID: PMC1994655
- DOI: 10.1016/j.drugalcdep.2007.02.008
Bupropion SR for the treatment of smokeless tobacco use
Abstract
Background: No pharmacotherapies have been shown to increase long-term (> or = 6 months) tobacco abstinence rates among smokeless tobacco (ST) users. Bupropion SR has demonstrated potential efficacy for ST users in pilot studies. We conducted a multicenter, randomized, double-blind, placebo-controlled, clinical trial to assess the efficacy and safety of bupropion SR for tobacco abstinence among ST users.
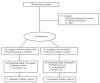
Methods: Adult ST users were randomized to bupropion SR titrated to 150 mg twice daily (N=113) or placebo (N=112) for 12 weeks plus behavioral intervention. The primary endpoint was the 7-day point-prevalence tobacco abstinence rate at week 12. Secondary outcomes included prolonged and continuous tobacco abstinence rates, craving and nicotine withdrawal, and weight gain.
Results: The 7-day point-prevalence tobacco abstinence rates did not differ between bupropion SR and placebo at the end treatment (53.1% versus 46.4%; odds ratio (OR) 1.3; p=0.301). The 7-day point-prevalence abstinence did not differ at weeks 24 and 52. The prolonged and continuous tobacco abstinence rates did not differ at weeks 12, 24, and 52. A time-by-treatment interaction was observed in craving over time with greater decreases in the bupropion SR group. At 12 weeks, the mean (+/-S.D.) weight change from baseline among abstinent subjects was an increase of 1.7 (+/-2.9)kg for the bupropion SR group compared to 3.2 (+/-2.7)kg for placebo (p=0.005).
Conclusions: Bupropion SR did not significantly increase tobacco abstinence rates among ST users, but it significantly decreased craving and weight gain over the treatment period.
Figures



References
-
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, Jacob PI, Jarvis MJ, LeHouezec J, Lichtenstein E, Tsoh J, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. - PubMed
-
- Boyle RG, Jensen J, Hatsukami DK, Severson HH. Measuring dependence in smokeless tobacco users. Addict Behav. 1995;20:443–450. - PubMed
-
- Dale LC, Ebbert JO, Schroeder DR, Croghan IT, Rasmussen DF, Trautman JA, Cox LS, Hurt RD. Bupropion for the treatment of nicotine dependence in spit tobacco users: a pilot study. Nicotine Tob Res. 2002;4:267–274. - PubMed
-
- Ebbert JO, Dale LC, Patten CA, Croghan IT, Schroeder DR, Moyer TP, Hurt RD. Effect of high dose nicotine patch therapy on tobacco withdrawal symptoms among smokeless tobacco users. Nicotine Tob Res. In press. - PubMed
-
- Ebbert JO, Klinkhammer MD, Stevens SR, Rowland LC, Offord KP, Ames SC, Dale LC. A survey of characteristics of smokeless tobacco users in a treatment program. Am J Health Behav. 2005;29:25–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

