Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture
- PMID: 17353237
- PMCID: PMC1855576
- DOI: 10.1128/AAC.01129-06
Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture
Abstract
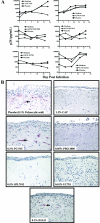
A human cervical explant culture was utilized for the preclinical assessment of anti-human immunodeficiency virus type 1 (HIV-1) activity and tissue toxicity of formulated, candidate topical microbicides. Products tested included cellulose acetate 1,2-benzene dicarboxylate (CAP), a carrageenan-based product (PC-515), a naphthalene sulfonate polymer (PRO 2000), a lysine dendrimer (SPL7013), a nonnucleoside reverse transcriptase inhibitor (UC781), and an antimicrobial peptide (D2A21), along with their placebos. Cervical explants were cultured overnight with HIV-1 with or without product, washed, and monitored for signs of HIV-1 infection. HIV-1 infection was determined by p24gag levels in the basolateral medium and by immunohistochemical analysis of the explant. Product toxicity was measured by the MTT [1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan] assay and histology. CAP, PRO 2000, SPL7013, and UC781 consistently prevented HIV-1 infection in all explants tested. PC-515 and D2A21 prevented HIV-1 infection in 50% or fewer of the explants tested. Placebos did not prevent infection in any of the explants tested. With the exception of PRO 2000 (4%), the MTT assay and histological analysis of the other products and placebos showed minimal toxicity to the epithelium and submucosa. Collectively, these data suggest that this culture system can be used for evaluating the safety and efficacy of topical microbicides designed for vaginal use.
Figures




References
-
- Abner, S. R., P. C. Guenthner, J. Guarner, K. A. Hancock, J. E. Cummins, Jr., A. Fink, G. T. Gilmore, C. Staley, A. Ward, O. Ali, S. Binderow, S. Cohen, L. A. Grohskopf, L. Paxton, C. E. Hart, and C. S. Dezzutti. 2005. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J. Infect. Dis. 1921545-1556. - PubMed
-
- Bader, J. P., J. B. McMahon, R. J. Schultz, V. L. Narayanan, J. B. Pierce, W. A. Harrison, O. S. Weislow, C. F. Midelfort, S. F. Stinson, and M. R. Boyd. 1991. Oxathiin carboxanilide, a potent inhibitor of human immunodeficiency virus reproduction. Proc. Natl. Acad. Sci. USA 886740-6744. - PMC - PubMed
-
- Balzarini, J., L. Naesens, E. Verbeken, M. Laga, L. Van Damme, M. Parniak, L. Van Mellaert, J. Anne, and E. De Clercq. 1998. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 121129-1138. - PubMed
-
- Beer, B. E., and J. E. Cummins, Jr. 2005. Novel strategies in HIV prevention—development of topical microbicides, p. 277-290. In A. M. Doherty (ed.), Annual reports in medicinal chemistry, vol. 40. Academic Press, San Diego, CA.
-
- Beer, B. E., G. F. Doncel, F. C. Krebs, R. J. Shattock, P. S. Fletcher, R. W. Buckheit, Jr., K. Watson, C. S. Dezzutti, J. E. Cummins, E. Bromley, N. Richardson-Harman, L. A. Pallansch, C. Lackman-Smith, C. Osterling, M. Mankowski, S. R. Miller, B. J. Catalone, P. A. Welsh, M. K. Howett, B. Wigdahl, J. A. Turpin, and P. Reichelderfer. 2006. In vitro preclinical testing of nonoxynol-9 as potential anti-human immunodeficiency virus microbicide: a retrospective analysis of results from five laboratories. Antimicrob. Agents Chemother. 50713-723. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

