Covalent dimer species of beta-defensin Defr1 display potent antimicrobial activity against multidrug-resistant bacterial pathogens
- PMID: 17353239
- PMCID: PMC1855538
- DOI: 10.1128/AAC.01531-06
Covalent dimer species of beta-defensin Defr1 display potent antimicrobial activity against multidrug-resistant bacterial pathogens
Abstract
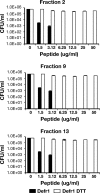
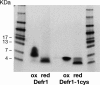
Beta defensins comprise a family of cationic, cysteine-rich antimicrobial peptides, predominantly expressed at epithelial surfaces. Previously we identified a unique five-cysteine defensin-related peptide (Defr1) that, when synthesized, is a mixture of dimeric isoforms and exhibits potent antimicrobial activity against Escherichia coli and Pseudomonas aeruginosa. Here we report that Defr1 displays antimicrobial activity against an extended panel of multidrug-resistant nosocomial pathogens for which antimicrobial treatment is limited or nonexistent. Defr1 fractions were collected by high-pressure liquid chromatography and analyzed by gel electrophoresis and mass spectrometry. Antimicrobial activity was initially investigated with the type strain Pseudomonas aeruginosa PAO1. All fractions tested displayed equivalent, potent antimicrobial activity levels comparable with that of the unfractionated Defr1. However, use of an oxidized, monomeric six-cysteine analogue (Defr1 Y5C), or of reduced Defr1, gave diminished antimicrobial activity. These results suggest that the covalent dimer structure of Defr1 is crucial to antimicrobial activity; this hypothesis was confirmed by investigation of a synthetic one-cysteine variant (Defr1-1cys). This gave an activity profile similar to that of synthetic Defr1 but only in an oxidized, dimeric form. Thus, we have shown that covalent, dimeric molecules based on the Defr1 beta-defensin sequence demonstrate antimicrobial activity even in the absence of the canonical cysteine motif.
Figures

References
-
- Campopiano, D. J., D. J. Clarke, N. C. Polfer, P. E. Barran, R. J. Langley, J. R. Govan, A. Maxwell, and J. R. Dorin. 2004. Structure-activity relationships in defensin dimers: a novel link between beta-defensin tertiary structure and antimicrobial activity. J. Biol. Chem. 27948671-48679. - PubMed
-
- Klüver, E., S. Schulz-Maronde, S. Scheid, B. Meyer, W. G. Forssmann, and K. Adermann. 2005. Structure-activity relation of human beta-defensin 3: influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity. Biochemistry 449804-9816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

