trans Autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining
- PMID: 17353268
- PMCID: PMC1899996
- DOI: 10.1128/MCB.02366-06
trans Autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining
Abstract
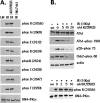
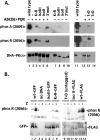
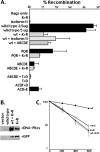
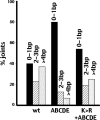
Recent studies have established that DNA-dependent protein kinase (DNA-PK) undergoes a series of autophosphorylation events that facilitate successful completion of nonhomologous DNA end joining. Autophosphorylation at sites in two distinct clusters regulates DNA end access to DNA end-processing factors and to other DNA repair pathways. Autophosphorylation within the kinase's activation loop regulates kinase activity. Additional autophosphorylation events (as yet undefined) occur that mediate kinase dissociation. Here we provide the first evidence that autophosphorylation within the two major clusters (regulating end access) occurs in trans. Further, both UV-induced and double-strand break (DSB)-induced phosphorylation in the two major clusters is predominantly autophosphorylation. Finally, we show that while autophosphorylation in trans on one of two synapsed DNA-PK complexes facilitates appropriate end processing, this is not sufficient to promote efficient end joining. This suggests that end joining in living cells requires additional phosphorylation events that either occur in cis or that occur on both sides of the DNA-PK synapse. These data support an emerging consensus that, via a series of autophosphorylation events, DNA-PK undergoes a sequence of conformational changes that promote efficient and appropriate repair of DSBs.
Figures

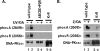
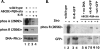
Similar articles
-
Autophosphorylation of DNA-dependent protein kinase regulates DNA end processing and may also alter double-strand break repair pathway choice.Mol Cell Biol. 2005 Dec;25(24):10842-52. doi: 10.1128/MCB.25.24.10842-10852.2005. Mol Cell Biol. 2005. PMID: 16314509 Free PMC article.
-
The DNA-dependent protein kinase catalytic subunit is phosphorylated in vivo on threonine 3950, a highly conserved amino acid in the protein kinase domain.Mol Cell Biol. 2007 Mar;27(5):1581-91. doi: 10.1128/MCB.01962-06. Epub 2006 Dec 11. Mol Cell Biol. 2007. PMID: 17158925 Free PMC article.
-
DNA-PK: the means to justify the ends?Adv Immunol. 2008;99:33-58. doi: 10.1016/S0065-2776(08)00602-0. Adv Immunol. 2008. PMID: 19117531 Review.
-
Nonhomologous end-joining deficiency of L5178Y-S cells is not associated with mutation in the ABCDE autophosphorylation cluster.Acta Biochim Pol. 2006;53(1):233-6. Epub 2006 Jan 9. Acta Biochim Pol. 2006. PMID: 16404475
-
DNA-dependent protein kinase in nonhomologous end joining: a lock with multiple keys?J Cell Biol. 2007 Oct 22;179(2):183-6. doi: 10.1083/jcb.200705106. Epub 2007 Oct 15. J Cell Biol. 2007. PMID: 17938249 Free PMC article. Review.
Cited by
-
Structural basis of long-range to short-range synaptic transition in NHEJ.Nature. 2021 May;593(7858):294-298. doi: 10.1038/s41586-021-03458-7. Epub 2021 Apr 14. Nature. 2021. PMID: 33854234 Free PMC article.
-
DNA repair pathways and their roles in drug resistance for lung adenocarcinoma.Mol Biol Rep. 2021 Apr;48(4):3813-3825. doi: 10.1007/s11033-021-06314-z. Epub 2021 Apr 15. Mol Biol Rep. 2021. PMID: 33856604 Review.
-
Activation of DNA-PK by ionizing radiation is mediated by protein phosphatase 6.PLoS One. 2009;4(2):e4395. doi: 10.1371/journal.pone.0004395. Epub 2009 Feb 9. PLoS One. 2009. PMID: 19198648 Free PMC article.
-
Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage.J Clin Invest. 2019 Jul 16;129(10):4245-4260. doi: 10.1172/JCI127613. J Clin Invest. 2019. PMID: 31310591 Free PMC article.
-
Biphasic Functional Interaction between the Adenovirus E4orf4 Protein and DNA-PK.J Virol. 2019 May 1;93(10):e01365-18. doi: 10.1128/JVI.01365-18. Print 2019 May 15. J Virol. 2019. PMID: 30842317 Free PMC article.
References
-
- Chan, D. W., and S. P. Lees-Miller. 1996. The DNA-dependent protein kinase is inactivated by autophosphorylation of the catalytic subunit. J. Biol. Chem. 271:8936-8941. - PubMed
-
- Chen, B. P., N. Uematsu, J. Kobayashi, Y. Lerenthal, A. Krempler, H. Yajima, M. Lobrich, Y. Shiloh, and D. J. Chen. 2006. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J. Biol. Chem. 282:6582-6587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
