The peptidyl prolyl cis/trans isomerase FKBP38 determines hypoxia-inducible transcription factor prolyl-4-hydroxylase PHD2 protein stability
- PMID: 17353276
- PMCID: PMC1899990
- DOI: 10.1128/MCB.01324-06
The peptidyl prolyl cis/trans isomerase FKBP38 determines hypoxia-inducible transcription factor prolyl-4-hydroxylase PHD2 protein stability
Abstract
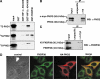
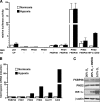
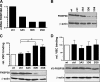
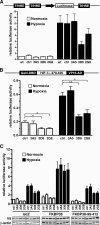
The heterodimeric hypoxia-inducible transcription factors (HIFs) are central regulators of the response to low oxygenation. HIF-alpha subunits are constitutively expressed but rapidly degraded under normoxic conditions. Oxygen-dependent hydroxylation of two conserved prolyl residues by prolyl-4-hydroxylase domain-containing enzymes (PHDs) targets HIF-alpha for proteasomal destruction. We identified the peptidyl prolyl cis/trans isomerase FK506-binding protein 38 (FKBP38) as a novel interactor of PHD2. Yeast two-hybrid, glutathione S-transferase pull-down, coimmunoprecipitation, colocalization, and mammalian two-hybrid studies confirmed specific FKBP38 interaction with PHD2, but not with PHD1 or PHD3. PHD2 and FKBP38 associated with their N-terminal regions, which contain no known interaction motifs. Neither FKBP38 mRNA nor protein levels were regulated under hypoxic conditions or after PHD inhibition, suggesting that FKBP38 is not a HIF/PHD target. Stable RNA interference-mediated depletion of FKBP38 resulted in increased PHD hydroxylation activity and decreased HIF protein levels and transcriptional activity. Reconstitution of FKBP38 expression abolished these effects, which were independent of the peptidyl prolyl cis/trans isomerase activity. Downregulation of FKBP38 did not affect PHD2 mRNA levels but prolonged PHD2 protein stability, suggesting that FKBP38 is involved in PHD2 protein regulation.
Figures


Similar articles
-
Hypoxia-inducible factor prolyl-4-hydroxylase PHD2 protein abundance depends on integral membrane anchoring of FKBP38.J Biol Chem. 2009 Aug 21;284(34):23046-58. doi: 10.1074/jbc.M109.032631. Epub 2009 Jun 22. J Biol Chem. 2009. PMID: 19546213 Free PMC article.
-
Oxygen sensing by the prolyl-4-hydroxylase PHD2 within the nuclear compartment and the influence of compartmentalisation on HIF-1 signalling.J Cell Sci. 2012 Nov 1;125(Pt 21):5168-76. doi: 10.1242/jcs.109041. Epub 2012 Sep 3. J Cell Sci. 2012. PMID: 22946054
-
Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase PHD2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness.Clin Cancer Res. 2006 Feb 15;12(4):1080-7. doi: 10.1158/1078-0432.CCR-05-2022. Clin Cancer Res. 2006. PMID: 16489060
-
HIF-prolyl hydroxylases and cardiovascular diseases.Toxicol Mech Methods. 2012 Jun;22(5):347-58. doi: 10.3109/15376516.2012.673088. Toxicol Mech Methods. 2012. PMID: 22424133 Review.
-
Role and regulation of prolyl hydroxylase domain proteins.Cell Death Differ. 2008 Apr;15(4):635-41. doi: 10.1038/cdd.2008.10. Epub 2008 Feb 15. Cell Death Differ. 2008. PMID: 18259202 Review.
Cited by
-
FKBP38 Regulates Self-Renewal and Survival of GBM Neurospheres.Cells. 2023 Nov 2;12(21):2562. doi: 10.3390/cells12212562. Cells. 2023. PMID: 37947640 Free PMC article.
-
The hypoxia-inducible factor pathway, prolyl hydroxylase domain protein inhibitors, and their roles in bone repair and regeneration.Biomed Res Int. 2014;2014:239356. doi: 10.1155/2014/239356. Epub 2014 May 11. Biomed Res Int. 2014. PMID: 24895555 Free PMC article. Review.
-
ZMYND10 functions in a chaperone relay during axonemal dynein assembly.Elife. 2018 Jun 19;7:e34389. doi: 10.7554/eLife.34389. Elife. 2018. PMID: 29916806 Free PMC article.
-
Prolyl hydroxylase domain protein 2 (PHD2) binds a Pro-Xaa-Leu-Glu motif, linking it to the heat shock protein 90 pathway.J Biol Chem. 2013 Apr 5;288(14):9662-9674. doi: 10.1074/jbc.M112.440552. Epub 2013 Feb 14. J Biol Chem. 2013. PMID: 23413029 Free PMC article.
-
The role of PHD2 mutations in the pathogenesis of erythrocytosis.Hypoxia (Auckl). 2014 Jul 1;2:71-90. doi: 10.2147/HP.S54455. eCollection 2014. Hypoxia (Auckl). 2014. PMID: 27774468 Free PMC article. Review.
References
-
- Appelhoff, R. J., Y. M. Tian, R. R. Raval, H. Turley, A. L. Harris, C. W. Pugh, P. J. Ratcliffe, and J. M. Gleadle. 2004. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279:38458-38465. - PubMed
-
- Bruick, R. K. 2003. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 17:2614-2623. - PubMed
-
- Bruick, R. K., and S. L. McKnight. 2001. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337-1340. - PubMed
-
- Bulgakov, O. V., J. T. Eggenschwiler, D. H. Hong, K. V. Anderson, and T. Li. 2004. FKBP8 is a negative regulator of mouse sonic hedgehog signaling in neural tissues. Development 131:2149-2159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
